myONE
GUDID 80726893120101
myONE measure your Treasure kit
GLOBAL PROTECTION CORP.
Basic male condom, Hevea-latex| Primary Device ID | 80726893120101 |
| NIH Device Record Key | f7b10ab9-e405-45f8-bc99-f78b2e752a71 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | myONE |
| Version Model Number | MY-MAPD25 |
| Company DUNS | 364614362 |
| Company Name | GLOBAL PROTECTION CORP. |
| Device Count | 25 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | true |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | 6179462800 |
| ccataldo@globalprotection.com | |
| Phone | 6179462800 |
| ccataldo@globalprotection.com | |
| Phone | 6179462800 |
| ccataldo@globalprotection.com | |
| Phone | 6179462800 |
| ccataldo@globalprotection.com | |
| Phone | 6179462800 |
| ccataldo@globalprotection.com | |
| Phone | 6179462800 |
| ccataldo@globalprotection.com | |
| Phone | 6179462800 |
| ccataldo@globalprotection.com | |
| Phone | 6179462800 |
| ccataldo@globalprotection.com | |
| Phone | 6179462800 |
| ccataldo@globalprotection.com | |
| Phone | 6179462800 |
| ccataldo@globalprotection.com | |
| Phone | 6179462800 |
| ccataldo@globalprotection.com | |
| Phone | 6179462800 |
| ccataldo@globalprotection.com | |
| Phone | 6179462800 |
| ccataldo@globalprotection.com | |
| Phone | 6179462800 |
| ccataldo@globalprotection.com | |
| Phone | 6179462800 |
| ccataldo@globalprotection.com |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Store at room temperature in a dark, dry place. Avoid excessive heat. |
| Special Storage Condition, Specify | Between 0 and 0 *Store at room temperature in a dark, dry place. Avoid excessive heat. |
| Special Storage Condition, Specify | Between 0 and 0 *Store at room temperature in a dark, dry place. Avoid excessive heat. |
| Special Storage Condition, Specify | Between 0 and 0 *Store at room temperature in a dark, dry place. Avoid excessive heat. |
| Special Storage Condition, Specify | Between 0 and 0 *Store at room temperature in a dark, dry place. Avoid excessive heat. |
| Special Storage Condition, Specify | Between 0 and 0 *Store at room temperature in a dark, dry place. Avoid excessive heat. |
| Special Storage Condition, Specify | Between 0 and 0 *Store at room temperature in a dark, dry place. Avoid excessive heat. |
| Special Storage Condition, Specify | Between 0 and 0 *Store at room temperature in a dark, dry place. Avoid excessive heat. |
| Special Storage Condition, Specify | Between 0 and 0 *Store at room temperature in a dark, dry place. Avoid excessive heat. |
| Special Storage Condition, Specify | Between 0 and 0 *Store at room temperature in a dark, dry place. Avoid excessive heat. |
| Special Storage Condition, Specify | Between 0 and 0 *Store at room temperature in a dark, dry place. Avoid excessive heat. |
| Special Storage Condition, Specify | Between 0 and 0 *Store at room temperature in a dark, dry place. Avoid excessive heat. |
| Special Storage Condition, Specify | Between 0 and 0 *Store at room temperature in a dark, dry place. Avoid excessive heat. |
| Special Storage Condition, Specify | Between 0 and 0 *Store at room temperature in a dark, dry place. Avoid excessive heat. |
| Special Storage Condition, Specify | Between 0 and 0 *Store at room temperature in a dark, dry place. Avoid excessive heat. |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00726893120105 [Primary] |
| GS1 | 80726893120101 [Unit of Use] |
FDA Product Code
| HIS | Condom |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2025-01-29 |
| Device Publish Date | 2025-01-21 |
On-Brand Devices [myONE]
| 80726893120101 | myONE measure your Treasure kit |
| 20726893120086 | MyONE Super Wide 64K, 10-count |
| 20726893120147 | A lubricated latex condoms |
Trademark Results [myONE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MYONE 98282835 not registered Live/Pending |
Chen, Xinzhou 2023-11-22 |
 MYONE 98099913 not registered Live/Pending |
ONEUNITED BANK 2023-07-25 |
 MYONE 97031691 not registered Live/Pending |
Life Technologies Corporation 2021-09-16 |
 MYONE 90209907 not registered Live/Pending |
Chen, Xinzhou 2020-09-25 |
 MYONE 79354401 not registered Live/Pending |
Motel One GmbH 2022-06-20 |
 MYONE 78460637 3604277 Dead/Cancelled |
Radio One, Inc. 2004-08-02 |
 MYONE 78460631 3551790 Dead/Cancelled |
Radio One, Inc. 2004-08-02 |
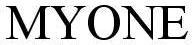 MYONE 78460629 3473630 Dead/Cancelled |
Radio One, Inc. 2004-08-02 |
 MYONE 78460628 not registered Dead/Abandoned |
Radio One, Inc. 2004-08-02 |
 MYONE 78460624 not registered Dead/Abandoned |
Radio One, Inc. 2004-08-02 |
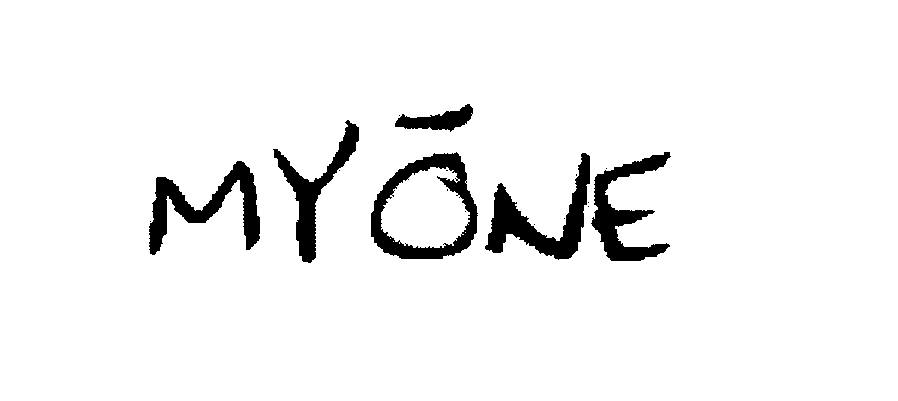 MYONE 76059203 not registered Dead/Abandoned |
Strickland, Joe D. 2000-06-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.