SmartVest SV-20-SS-PK
GUDID B102060078S0040
LTU SmartVest® – Child S-Small (Pink)
ELECTROMED, INC.
Chest-oscillation airway secretion-clearing system vest| Primary Device ID | B102060078S0040 |
| NIH Device Record Key | 2b5e75f5-e510-4cba-a682-67e6cbce7d55 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | SmartVest |
| Version Model Number | SV-20-SS-PK |
| Catalog Number | SV-20-SS-PK |
| Company DUNS | 036514250 |
| Company Name | ELECTROMED, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | B102060078S0040 [Primary] |
FDA Product Code
| BYI | Percussor, Powered-Electric |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2019-12-11 |
| Device Publish Date | 2016-09-16 |
On-Brand Devices [SmartVest]
| B10209488S1510 | Refurb SQL® SmartVest®, Wireless (Red) |
| B102090740S8400 | SQL-W Supplement Kit (USA) |
| B102090683S0100 | Mobile Pedestal - SQL® |
| B102090649S0800 | SQL-I Supplement Kit (Hong Kong) |
| B102090649S0700 | SQL-I Supplement Kit (Mexico) |
| B102090649S0600 | SQL-I Supplement Kit (Canada) |
| B102090649S0500 | SQL-I Supplement Kit (Taiwan) |
| B102090649S0400 | SQL-I Supplement Kit (Germany) |
| B102090649S0300 | SQL-I Supplement Kit (Italy) |
| B102090649S0200 | SQL-I Supplement Kit (Spain) |
| B102090649S0100 | SQL-I Supplement Kit (USA) |
| B102090579S0100 | SQL® Replacement Filters - 2-Pack |
| B102090535S0100 | SQL® Filter & Fuse Maintenance Kit |
| B102090504S0100 | Power Cord - Class II, North America |
| B102090499S8400 | SQL® Supplement Kit (USA) |
| B102090488S1500 | SQL® SmartVest® ACS,Wireless (Red) |
| B102090488S1410 | Refurb SQL® SmartVest®, Wireless (BT BL) |
| B102090488S1400 | SQL® SmartVest® ACS,Wireless (Bright BL) |
| B102090488S1310 | Refurb SQL® SmartVest®, Wireless (Blue) |
| B102090488S1300 | SQL® SmartVest® ACS,Wireless (Blue) |
| B102090488S1110 | Refurb SQL® SmartVest®, Wireless |
| B102090488S1100 | SQL® SmartVest® ACS,Wireless |
| B102090488S0510 | Refurbished SQL® SmartVest® ACS (Red) |
| B102090488S0500 | SQL® SmartVest® ACS (Red) |
| B102090488S0410 | Refurbished SQL® SmartVest® ACS (BT Blue) |
| B102090488S0400 | SQL® SmartVest® ACS (Bright Blue) |
| B102090488S0310 | Refurbished SQL® SmartVest® ACS (Blue) |
| B102090488S0300 | SQL® SmartVest® ACS (Blue) |
| B102090488S0210 | Refurbished SQL-I SmartVest® ACS |
| B102090488S0200 | SQL-I SmartVest® ACS |
| B102090488S0110 | Refurbished SQL® SmartVest® ACS |
| B102090488S0100 | SQL® SmartVest® ACS |
| B102070716S0100 | Power Cord – North America |
| B102070686S0100 | Mobile Pedestal |
| B102070622S0100 | Refurbished Mobile Pedestal - SV2100-I |
| B102070172S0200 | LTU Connecting Hose |
| B102070172S0100 | SPU Connecting Hose |
| B102070170S0100 | O-Ring Replacement Kit For Connecting Hose |
| B102070137S8400 | Supplement Kit (USA – Institutional) |
| B102070137S1240 | Supplement Kit (Canada – Institutional) |
| B102070124S9990 | Supplement Kit (Custom International) |
| B102070124S8400 | Supplement Kit (USA) |
| B102070124S7920 | Supplement Kit (Turkey) |
| B102070124S7840 | Supplement Kit (United Arab Emirates) |
| B102070124S4840 | Supplement Kit (Mexico) |
| B102070124S4100 | Supplement Kit (Republic of Korea) |
| B102070124S3920 | Supplement Kit (Japan) |
| B102070124S3440 | Supplement Kit (Hong Kong)) |
| B102070124S1580 | Supplement Kit (Taiwan) |
| B102070124S1240 | Supplement Kit (Canada) |
Trademark Results [SmartVest]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SMARTVEST 98475019 not registered Live/Pending |
Achieva Credit Union 2024-03-29 |
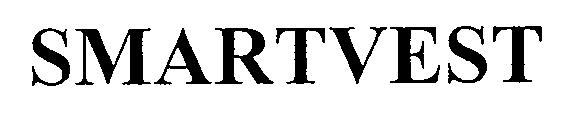 SMARTVEST 76705775 4137272 Live/Registered |
ELECTROMED, INC. 2010-12-23 |
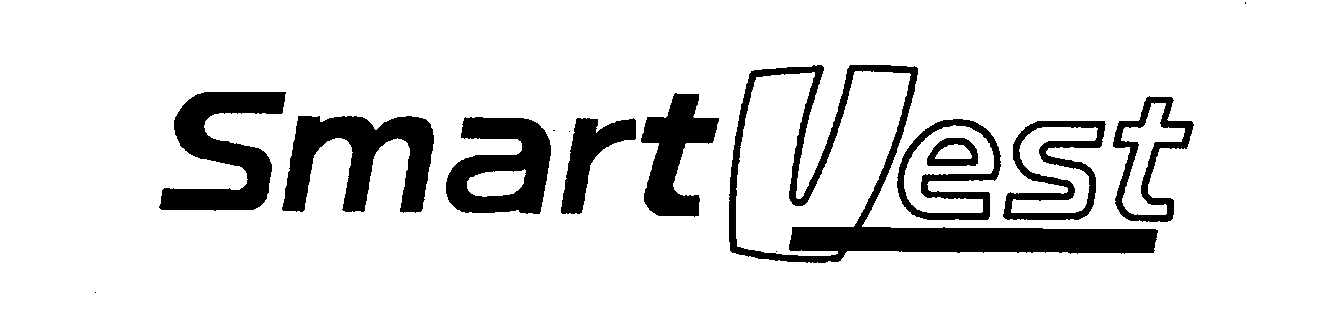 SMARTVEST 76672340 3424816 Dead/Cancelled |
ELECTROMED, INC. 2007-02-07 |
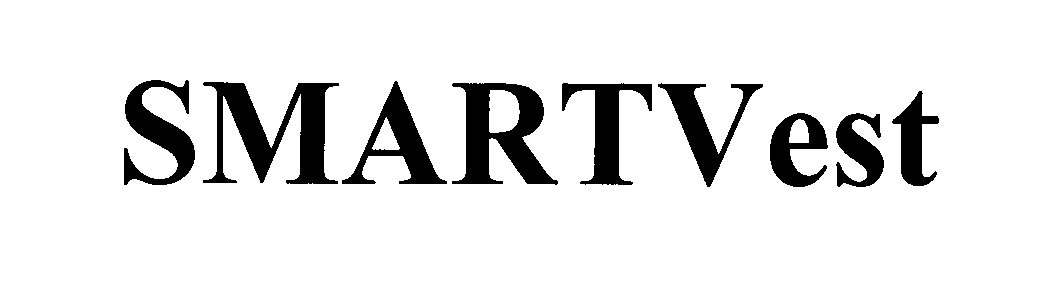 SMARTVEST 76585852 3016390 Live/Registered |
ELECTROMED, INC. 2004-04-05 |
 SMARTVEST 73775995 not registered Dead/Abandoned |
BROKER DEALER SERVICES, INC. 1989-01-23 |
 SMARTVEST 73645122 1463080 Dead/Cancelled |
OLDE DISCOUNT CORPORATION 1987-02-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.