Mark Image
Registration | Serial | Company Trademark
Application Date |
|---|
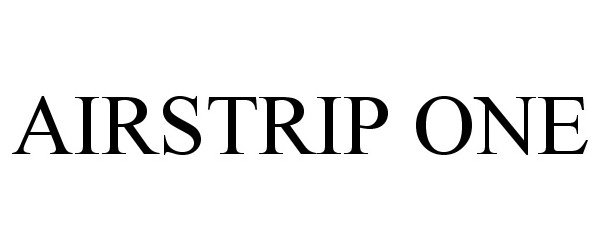 AIRSTRIP ONE 86273553 5386889 Live/Registered |
AirStrip IP Holdings, LLC 2014-05-06 |
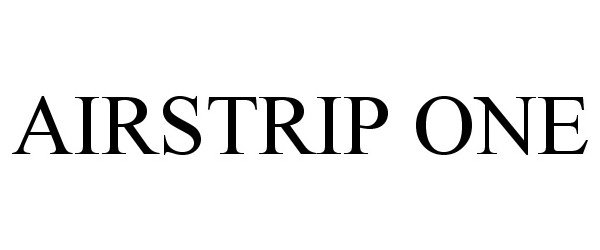 AIRSTRIP ONE 86273550 5386887 Live/Registered |
AirStrip IP Holdings, LLC 2014-05-06 |
 AIRSTRIP ONE 86073057 5335621 Live/Registered |
AirStrip IP Holdings, LLC 2013-09-24 |
 AIRSTRIP ONE 86073051 4773491 Live/Registered |
AirStrip IP Holdings, LLC 2013-09-24 |
 AIRSTRIP ONE 86073050 4773490 Live/Registered |
AirStrip IP Holdings, LLC 2013-09-24 |
 AIRSTRIP ONE 86073047 5329917 Live/Registered |
AirStrip IP Holdings, LLC 2013-09-24 |
 AIRSTRIP ONE 86073042 4676285 Live/Registered |
AirStrip IP Holdings, LLC 2013-09-24 |
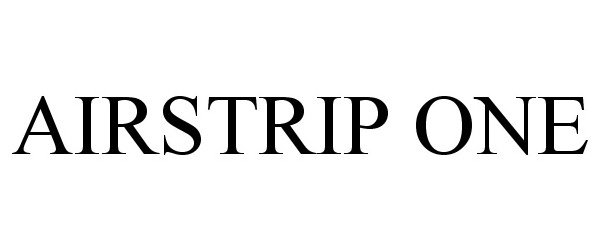 AIRSTRIP ONE 85753316 4433758 Live/Registered |
AirStrip IP Holdings, LLC 2012-10-12 |
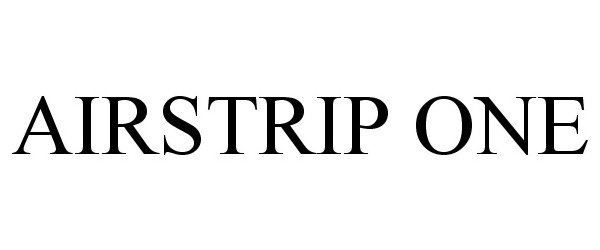 AIRSTRIP ONE 85753312 4522783 Live/Registered |
AirStrip IP Holdings, LLC 2012-10-12 |
 AIRSTRIP ONE 85753310 4433757 Live/Registered |
AirStrip IP Holdings, LLC 2012-10-12 |
 AIRSTRIP ONE 85753307 4433756 Live/Registered |
AirStrip IP Holdings, LLC 2012-10-12 |
 AIRSTRIP ONE 85753306 4433755 Live/Registered |
AirStrip IP Holdings, LLC 2012-10-12 |











