2-CAN™ 103-1-03
GUDID B2671031030
6.5mm 2-CAN (4.8mm Outer Diameter)
Flow-FX
Orthopaedic coaxial injection cannula| Primary Device ID | B2671031030 |
| NIH Device Record Key | 8d1697aa-504e-4eab-9b7e-6f3a44d153ae |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | 2-CAN™ |
| Version Model Number | 103-1-03 |
| Catalog Number | 103-1-03 |
| Company DUNS | 079293587 |
| Company Name | Flow-FX |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Dimensions
| Outer Diameter | 4.8 Millimeter |
| Outer Diameter | 4.8 Millimeter |
| Outer Diameter | 4.8 Millimeter |
| Outer Diameter | 4.8 Millimeter |
| Outer Diameter | 4.8 Millimeter |
| Outer Diameter | 4.8 Millimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | B2671031030 [Primary] |
FDA Product Code
| GEA | Cannula, Surgical, General & Plastic Surgery |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2019-12-24 |
| Device Publish Date | 2019-12-16 |
On-Brand Devices [2-CAN™]
| B2671031050 | 8mm 2-CAN™ 350mm |
| B2671031040 | 8mm 2-CAN™ 450mm |
| B2671031030 | 6.5mm 2-CAN (4.8mm Outer Diameter) |
| B2671031020 | 5.5mm 2-CAN (3.6mm Outer Diameter) |
| B2671035051 | 2-CAN 8mm Short Carton |
| B2671035041 | 2-CAN 8mm Long Carton |
| B26710310052 | 2-CAN 8mm Short Shipper |
| B26710310042 | 2-CAN 8mm Long Shipper |
| B2672CAN450B0 | 2-CAN™ 450mm Delivery Cannula |
| B2672CAN350B0 | 2-CAN™ 350mm Delivery Cannula |
| B2672CAN3500 | 2-CAN™ 350mm Delivery Cannula |
Trademark Results [2-CAN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
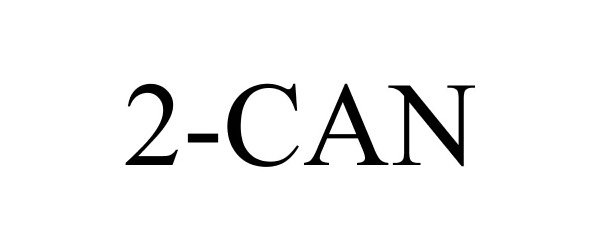 2-CAN 86155150 5027489 Live/Registered |
Orthopedic Generations LLC 2013-12-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.