Scout 82389
GUDID B314823891
Power Wheelchair
BURKE, INC.
Wheelchair, electric-motor-driven, attendant/occupant-controlled, powered-steering, non-collapsible| Primary Device ID | B314823891 |
| NIH Device Record Key | 0ac1a0be-405a-4efb-be96-2ed877823ca0 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Scout |
| Version Model Number | Boss 4.5 TPB |
| Catalog Number | 82389 |
| Company DUNS | 045093101 |
| Company Name | BURKE, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 913-722-5658 |
| jernst@burke-mobility.com | |
| Phone | 913-722-5658 |
| jernst@burke-mobility.com | |
| Phone | 913-722-5658 |
| jernst@burke-mobility.com | |
| Phone | 913-722-5658 |
| jernst@burke-mobility.com | |
| Phone | 913-722-5658 |
| jernst@burke-mobility.com | |
| Phone | 913-722-5658 |
| jernst@burke-mobility.com | |
| Phone | 913-722-5658 |
| jernst@burke-mobility.com | |
| Phone | 913-722-5658 |
| jernst@burke-mobility.com | |
| Phone | 913-722-5658 |
| jernst@burke-mobility.com | |
| Phone | 913-722-5658 |
| jernst@burke-mobility.com | |
| Phone | 913-722-5658 |
| jernst@burke-mobility.com | |
| Phone | 913-722-5658 |
| jernst@burke-mobility.com | |
| Phone | 913-722-5658 |
| jernst@burke-mobility.com | |
| Phone | 913-722-5658 |
| jernst@burke-mobility.com | |
| Phone | 913-722-5658 |
| jernst@burke-mobility.com | |
| Phone | 913-722-5658 |
| jernst@burke-mobility.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | B314823891 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K023961
FDA Product Code
| ITI | Wheelchair, Powered |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-09-01 |
On-Brand Devices [Scout]
| B314831821 | Power Wheelchair |
| B314831811 | Power Wheelchair |
| B314831801 | Power Wheelchair |
| B314831731 | Power Wheelchair |
| B314826771 | Power Wheelchair |
| B314826761 | Power Wheelchair |
| B314826471 | Power Wheelchair |
| B314826281 | Power Wheelchair |
| B314825541 | Power Wheelchair |
| B314824911 | Power Wheelchair |
| B314823891 | Power Wheelchair |
| B314823481 | Power Wheelchair |
| B314823381 | Power Wheelchair |
| B314823061 | Power Wheelchair |
| B314820621 | Boss 6.75 |
| B31481855A1 | Power Wheelchair |
| B31481445A1 | Power Wheelchair |
| B314811221 | Power Wheelchair |
| B31481113A1 | Power Wheelchair |
| B313822501 | Power Wheelchair |
Trademark Results [Scout]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SCOUT 98875269 not registered Live/Pending |
Tutan, LLC 2024-11-27 |
 SCOUT 98863661 not registered Live/Pending |
Turbo Drill Industries, Inc. 2024-11-20 |
 SCOUT 98780580 not registered Live/Pending |
Revir Technologies, Inc. 2024-10-01 |
 SCOUT 98761899 not registered Live/Pending |
Scout Motors Inc. 2024-09-20 |
 SCOUT 98749729 not registered Live/Pending |
Scout Trading Co. LLC 2024-09-13 |
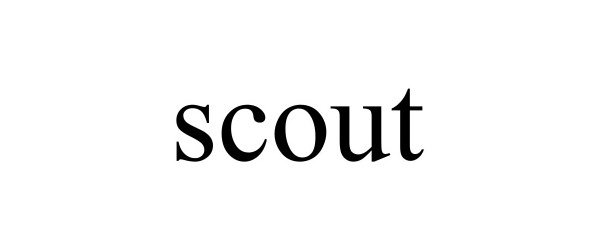 SCOUT 98652573 not registered Live/Pending |
Insurtech Ohio LLC 2024-07-17 |
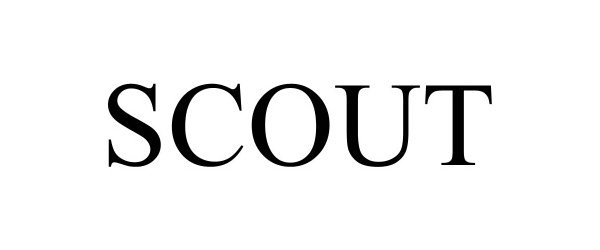 SCOUT 98636640 not registered Live/Pending |
International Hunter Education Association - USA 2024-07-08 |
 SCOUT 98636315 not registered Live/Pending |
Price Industries Limited 2024-07-08 |
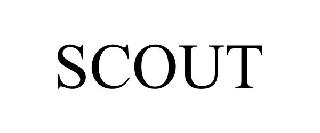 SCOUT 98573972 not registered Live/Pending |
Adventurer Mfg. Limited Partnership 2024-05-29 |
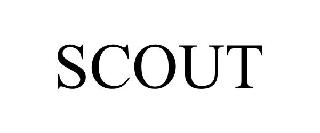 SCOUT 98573959 not registered Live/Pending |
Adventurer Mfg. Limited Partnership 2024-05-29 |
 SCOUT 98557411 not registered Live/Pending |
Fusion Trade, Inc. 2024-05-17 |
 SCOUT 98548024 not registered Live/Pending |
Quantum Space, LLC 2024-05-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.