PCRopsis 783946025
GUDID B5507839461000
PCRopsis™ Reagent RVD-WHO is an alternative to viral RNA extraction from nasopharyngeal or oropharyngeal swab samples transported in WHO transport medium.
ENTOPSIS, LLC
Nucleic acid extraction/isolation kit IVD| Primary Device ID | B5507839461000 |
| NIH Device Record Key | 9cdc720b-b294-4f6b-a96a-b3e610baa1ab |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | PCRopsis |
| Version Model Number | Reagent RVD-WHO |
| Catalog Number | 783946025 |
| Company DUNS | 078294784 |
| Company Name | ENTOPSIS, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | B5507839461000 [Primary] |
FDA Product Code
| JJH | Clinical Sample Concentrator |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-09-16 |
| Device Publish Date | 2020-09-08 |
On-Brand Devices [PCRopsis]
| B5508880200 | PCRopsis™ Universal Viral Transport is intended for the collection and non-propagating transpo |
| B5508880050 | PCRopsis™ Universal Viral Transport is intended for the collection and non-propagating transpo |
| B5508880010 | PCRopsis™ Universal Viral Transport is intended for the collection and non-propagating transpo |
| B5507839461000 | PCRopsis™ Reagent RVD-WHO is an alternative to viral RNA extraction from nasopharyngeal or oro |
| B5507839460250 | PCRopsis™ Reagent RVD-WHO is an alternative to viral RNA extraction from nasopharyngeal or oro |
| B5507831000 | PCRopsis™ Reagent RVD is an alternative to viral RNA extraction from nasopharyngeal or orophar |
| B5507830250 | PCRopsis™ Reagent RVD is an alternative to viral RNA extraction from nasopharyngeal or orophar |
| B550888100158 | PCRopsis™ Universal Viral Transport (UVT) is intended for the collection and non-propagating t |
| B55088800003 | PCRopsis™ Universal Viral Transport (UVT) is intended for the collection and non-propagating t |
| B55078335000 | PCRopsis™ Reagent RVD-E mediates extraction-free RT-qPCR of specimens directly from swabs, wit |
| B55078331000 | PCRopsis™ Reagent RVD-E mediates extraction-free RT-qPCR of specimens directly from swabs, wit |
| B55078330250 | PCRopsis™ Reagent RVD-E mediates extraction-free RT-qPCR of specimens directly from swabs, wit |
| B55078310000 | PCRopsis™ Reagent RVD mediates extraction-free RT-qPCR of swab specimens transported in compat |
| B5507833641000 | PCRopsis™ RVD Enhancer (100 mL) is intended to enhance PCR-mediated amplification of gene targ |
| B5507833640100 | PCRopsis™ RVD Enhancer is intended to enhance PCR-mediated amplification of gene targets when |
| B550778310000 | PCRopsis™ Reagent SRVD (1000 mL) mediates extraction-free RT-qPCR from specimens in saliva. |
| B55077831000 | PCRopsis™ Reagent SRVD (100 mL) mediates extraction-free RT-qPCR from specimens in saliva. |
| B55077830250 | PCRopsis™ Reagent SRVD (25 mL) mediates extraction-free RT-qPCR from specimens in saliva. |
| B5502825000 | PCRopsis™ Reagent Buccal (500 mL) mediates extraction-free PCR from buccal specimens directly |
| B55028210000 | PCRopsis™ Reagent Buccal (1,000 mL) mediates extraction-free PCR from buccal specimens directl |
| B5502821000 | PCRopsis™ Reagent Buccal (100 mL) mediates extraction-free PCR from buccal specimens directly |
| B5502820250 | PCRopsis™ Reagent Buccal (25 mL) mediates extraction-free PCR from buccal specimens directly f |
| B5507837810000 | PCRopsis™ Reagent RVD-RT (1000 mL) mediates extraction-free PCR from saliva, urine, and swab s |
| B550783781000 | PCRopsis™ Reagent RVD-RT (100 mL) mediates extraction-free PCR from saliva, urine, and swab sp |
| B550783780250 | PCRopsis™ Reagent RVD-RT (25 mL) mediates extraction-free PCR from saliva, urine, and swab spe |
| B55026685036 | PCRopsis™ Concentrator (50 pre-loaded tubes) concentrates clinical samples. |
| B550266825047 | PCRopsis™ Concentrator (250 pre-loaded tubes) concentrates clinical samples. |
| B550227610000 | PCRopsis™ BCS Nano (1000 mL) is intended for selective precipitation of red blood cells from w |
| B55022761000 | PCRopsis™ BCS Nano (100 mL) is intended for selective precipitation of red blood cells from wh |
| B55022760100 | PCRopsis™ BCS Nano (10 mL) is intended for selective precipitation of red blood cells from who |
| B5507870010 | PCRopsis™ Support helps mediate select Next Gen Direct PCR™ applications (e.g., PCR amplific |
| B5503585000 | PCRopsis™ Elution Buffer is intended to elute test material from substrates. |
| B55035810000 | PCRopsis™ Elution Buffer is intended to elute test material from substrates. |
| B5503581000 | PCRopsis™ Elution Buffer is intended to elute test material from substrates. |
| B5503580250 | PCRopsis™ Elution Buffer is intended to elute test material from substrates |
| B5502280010 | PCRopsis™ Activator helps mediate room temperature Next Gen Direct PCR™ |
| B5507833610000 | PCRopsis™ Reagent RVD with RVD Enhancer (1 L) mediates extraction-free RT-qPCR of various spec |
| B550783361000 | PCRopsis™ Reagent RVD with RVD Enhancer (100 mL) mediates extraction-free RT-qPCR of various s |
| B550783360250 | PCRopsis™ Reagent RVD with RVD Enhancer (25 mL) mediates extraction-free RT-qPCR of various sp |
| B55059785036 | PCRopsis™ Lysis Beads (50 pre-loaded tubes) facilitate lysis of difficult to lyse microorganis |
| B5507870020 | PCRopsis™ Support (0.25 mL) helps mediate select Next Gen Direct PCR™ applications (e.g., PC |
| B55059725046 | PCRopsis™ Lysis Beads (250 pre-loaded tubes) facilitate lysis of difficult to lyse microorgani |
| B55059700250 | PCRopsis™ Lysis Beads (25 grams, bulk) facilitate lysis of difficult to lyse microorganisms. |
| B55059705000 | PCRopsis™ Lysis Beads (500 grams, bulk) facilitate lysis of difficult to lyse microorganisms. |
| B5502280250 | PCRopsis™ Activator (25 mL) helps mediate room temperature Next Gen Direct PCR™ |
| B55059725000 | PCRopsis™ Lysis Beads (2.5 kilograms, bulk) facilitate lysis of difficult to lyse microorganis |
| B55059710000 | PCRopsis™ Lysis Beads (1 kilogram, bulk) facilitate lysis of difficult to lyse microorganisms. |
| B5507870040 | PCRopsis™ Support (500 uL) helps mediate select Next Gen Direct PCR™ applications (e.g., PCR |
| B55059700100 | PCRopsis™ Lysis Beads (10 grams, bulk) facilitate lysis of difficult to lyse microorganisms. |
| B55087810000 | PCRopsis™ UrineA (1 mL) improves the functionality of PCRopsis™ Reagent 123, especially when |
Trademark Results [PCRopsis]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
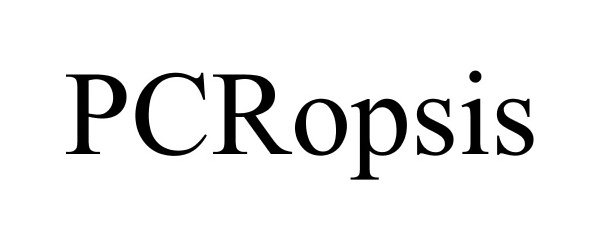 PCROPSIS 87891197 5641569 Live/Registered |
Entopsis LLC 2018-04-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.