HI-I 09032-001P
GUDID B71509032001P0
Special Grain #2 SF HI-I® LW
FRICKE INTERNATIONAL, INC.
Dental appliance fabrication material, cured| Primary Device ID | B71509032001P0 |
| NIH Device Record Key | dfcdb361-664e-4ce9-a93d-4c9c8cb1e837 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | HI-I |
| Version Model Number | 09032-001P |
| Catalog Number | 09032-001P |
| Company DUNS | 957630726 |
| Company Name | FRICKE INTERNATIONAL, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
| Weight | 1 Pound |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | B71509032001P0 [Primary] |
FDA Product Code
| EBI | Resin, Denture, Relining, Repairing, Rebasing |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-09-23 |
| Device Publish Date | 2020-09-15 |
On-Brand Devices [HI-I]
| B71513023025P0 | Special Fibered #1 "LE" HI-I® Pour |
| B71509033006P0 | Special Grain #3 SF HI-I® LW |
| B71513023006P0 | Special Fibered #1 "LE" HI-I® Pour |
| B71509032001P0 | Special Grain #2 SF HI-I® LW |
| B71509032006P0 | Special Grain #2 SF HI-I® LW |
Trademark Results [HI-I]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
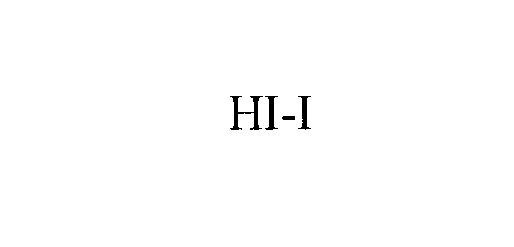 HI-I 76137619 2498241 Live/Registered |
Fricke, Lawrence R. Jr. 2000-09-29 |
 HI-I 73029310 1034625 Dead/Expired |
FRICKE, LAWRENCE R. 1974-08-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.