KLYO
GUDID B773KEOPT0
Mobile, operating tables (Electromechanical Operating Tables, Electrohydraulic Operating Tables) designed to be adjusted to support a patient during many types of surgical interventions. The table surface consists of many articulated sections that can be elevated or lowered for contouring to accommodate numerous anatomical positions to satisfy the requirements of many clinical specialties.
KLYO MEDICAL SYSTEMS INC.
Universal operating table, electromechanical| Primary Device ID | B773KEOPT0 |
| NIH Device Record Key | 9bc9399c-9ded-471a-a53d-26c3009e71ee |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | KLYO |
| Version Model Number | KEOPT |
| Company DUNS | 058639417 |
| Company Name | KLYO MEDICAL SYSTEMS INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | B773KEOPT0 [Primary] |
FDA Product Code
| FQO | Table, Operating-Room, Ac-Powered |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-02-29 |
| Device Publish Date | 2024-02-21 |
On-Brand Devices [KLYO]
| B773KLEDM0 | Single, Double and Triple M-Series LED Surgical Light system with and without Variable Color Tem |
| B773KLTEM77 | Mobile, electro- motorized table designed to be adjusted to support a patient during many types |
| B773KEOPT0 | Mobile, operating tables (Electromechanical Operating Tables, Electrohydraulic Operating Tables) |
| B773KMHBD0 | A mechanical bed designed to be used as a patient bed for rest/sleep in a hospital ward/room wit |
| B773KHSTR0 | A battery-powered mobile platform designed to transport a recumbent patient within a healthcare |
| B773KEHBD0 | A mains electricity (AC-powered) bed designed to be used as a patient bed for rest/sleep in a ho |
Trademark Results [KLYO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
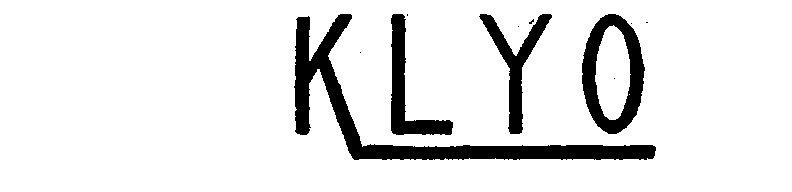 KLYO 71613895 0557391 Dead/Expired |
B & B ENGINEERING CORPORATION 1951-05-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.