ICON ICONBL-886
GUDID B958ICONBL887
GLOVES NITRILE EXAMINATION POWDER-FREE TEXTURED BLUE, FENTANYL TESTED ICONBL-887 MEDIUM
Excel Gloves & Safety Supplies, Inc.
Nitrile examination/treatment glove, non-powdered, non-antimicrobial| Primary Device ID | B958ICONBL887 |
| NIH Device Record Key | 13b81a84-927d-4656-8ee8-9925a5ffc824 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | ICON |
| Version Model Number | ICONBL-887 |
| Catalog Number | ICONBL-886 |
| Company DUNS | 928913409 |
| Company Name | Excel Gloves & Safety Supplies, Inc. |
| Device Count | 100 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com | |
| Phone | 2538961195 |
| glovelady@excelsupplycompany.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | B958ICONBL887 [Unit of Use] |
| HIBCC | B958ICONBL8871 [Primary] |
FDA Product Code
| QDO | Fentanyl And Other Opioid Protection Glove |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-08-02 |
| Device Publish Date | 2023-07-25 |
On-Brand Devices [ICON]
| B958ICONBL889 | GLOVES NITRILE EXAMINATION POWDER-FREE TEXTURES FENTANYL TESTED BLUE ICONBL-889 EXTRA LARGE (xl) |
| B958ICONBL888 | GLOVES NITRILEEXAMINATION POWDER-FREE TEXTURED FENTANYL TESTED BLUE ICONBL-888 LARGE |
| B958ICONBL887 | GLOVES NITRILE EXAMINATION POWDER-FREE TEXTURED BLUE, FENTANYL TESTED ICONBL-887 MEDIUM |
| B958ICONBL886 | GLOVES NITRILE EXAMINATION POWDER-FREE TEXTURED BLUE ICONBL-886 SMALL |
| B958ICONBL8810 | GLOVES NITRILE EXAMINATION POWDER-FREE TEXTURES FENTANYL TESTED BLUE ICONBL8810 EXTRA, EXTRA LAR |
| B9584886 | GLOVES, EXAMINATION POWDER FREE EXAM TEXTURED SMALL ICON4886 |
Trademark Results [ICON]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ICON 98887242 not registered Live/Pending |
ZuMedia Inc. 2024-12-05 |
 ICON 98708053 not registered Live/Pending |
Vandor Corporation 2024-08-20 |
 ICON 98593063 not registered Live/Pending |
SARL XPLORER 2024-06-10 |
 ICON 98578673 not registered Live/Pending |
Tyler Candle Company, L.L.C. 2024-05-31 |
 ICON 98548381 not registered Live/Pending |
Green, Jermon D 2024-05-13 |
 ICON 98508431 not registered Live/Pending |
UFP Industries, Inc. 2024-04-18 |
 ICON 98482609 not registered Live/Pending |
Icon Scientific Limited 2024-04-03 |
 ICON 98482528 not registered Live/Pending |
Icon Scientific Limited 2024-04-03 |
 ICON 98453341 not registered Live/Pending |
M.P.T. Racing Inc 2024-03-17 |
 ICON 98422018 not registered Live/Pending |
E. & J. GALLO WINERY 2024-02-26 |
 ICON 98311970 not registered Live/Pending |
True Temper Sports, Inc. 2023-12-13 |
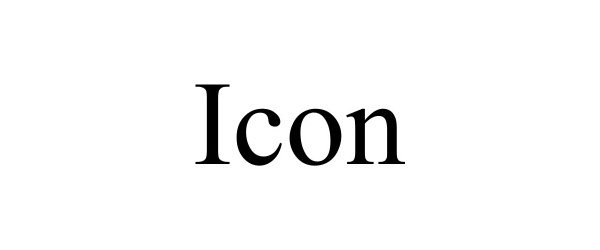 ICON 98275939 not registered Live/Pending |
Capital One Financial Corporation 2023-11-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.