BlackBox
GUDID BB4B963WS501C1
Cellular weight scale with a large platform and high weight capacity
Blackbox Remote Patient Monitoring LLC
Stand-on floor scale, electronic| Primary Device ID | BB4B963WS501C1 |
| NIH Device Record Key | 4cdb39de-a09a-4fac-9c03-71dc29744f08 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | BlackBox |
| Version Model Number | WS-501C |
| Company DUNS | 117652799 |
| Company Name | Blackbox Remote Patient Monitoring LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 4355129587 |
| Jared@BlackBoxRPM.com | |
| Phone | 4355129587 |
| Jared@BlackBoxRPM.com | |
| Phone | 4355129587 |
| Jared@BlackBoxRPM.com | |
| Phone | 4355129587 |
| Jared@BlackBoxRPM.com | |
| Phone | 4355129587 |
| Jared@BlackBoxRPM.com | |
| Phone | 4355129587 |
| Jared@BlackBoxRPM.com | |
| Phone | 4355129587 |
| Jared@BlackBoxRPM.com | |
| Phone | 4355129587 |
| Jared@BlackBoxRPM.com | |
| Phone | 4355129587 |
| Jared@BlackBoxRPM.com | |
| Phone | 4355129587 |
| Jared@BlackBoxRPM.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | B963WS501C0 [Primary] |
| HIBCC | BB4B963WS501C1 [Package] Contains: B963WS501C0 Package: Carton [4 Units] In Commercial Distribution |
FDA Product Code
| FRW | Scale, Patient |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-08-04 |
| Device Publish Date | 2022-07-27 |
On-Brand Devices [BlackBox]
| B963101C1 | Cellular Blood Pressure Monitor |
| BB4B963WS501C1 | Cellular weight scale with a large platform and high weight capacity |
Trademark Results [BlackBox]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
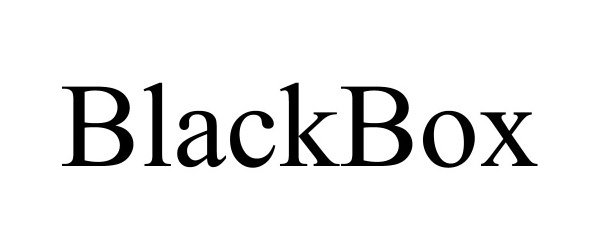 BLACKBOX 97300632 not registered Live/Pending |
The BlackBox Group LLC 2022-03-08 |
 BLACKBOX 97300573 not registered Live/Pending |
The BlackBox Group LLC 2022-03-08 |
 BLACKBOX 90728881 not registered Live/Pending |
Curtis, Jared Glen 2021-05-23 |
 BLACKBOX 90728881 not registered Live/Pending |
Shelley, Ean Cameron 2021-05-23 |
 BLACKBOX 90728881 not registered Live/Pending |
Shelley, Eric Joel 2021-05-23 |
 BLACKBOX 90728881 not registered Live/Pending |
Shelley, Joan E 2021-05-23 |
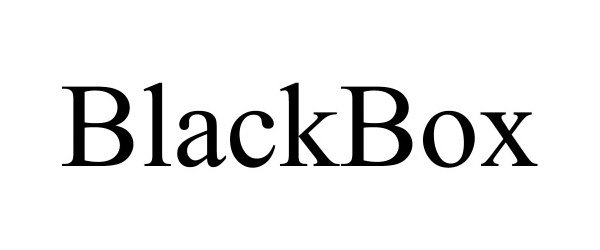 BLACKBOX 90728874 not registered Live/Pending |
Curtis, Jared Glen 2021-05-23 |
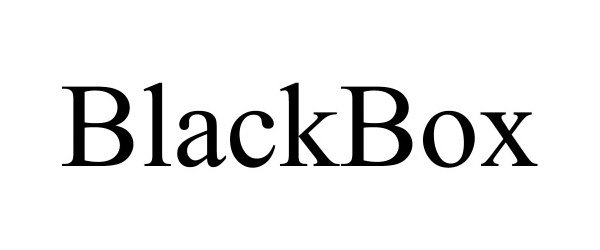 BLACKBOX 90728874 not registered Live/Pending |
Shelley, Ean Cameron 2021-05-23 |
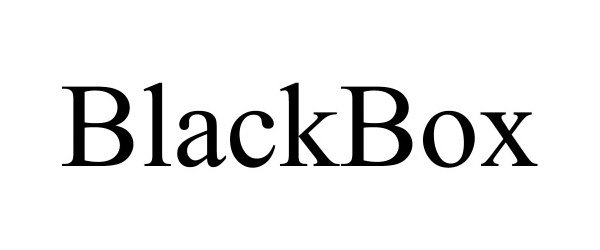 BLACKBOX 90728874 not registered Live/Pending |
Shelley, Eric Joel 2021-05-23 |
 BLACKBOX 90728874 not registered Live/Pending |
Shelley, Joan E 2021-05-23 |
 BLACKBOX 88775359 not registered Live/Pending |
Global Supplies Inc 2020-01-28 |
 BLACKBOX 88603553 not registered Live/Pending |
Blackbox Inc. 2019-09-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.