XIOS
GUDID D764B10700181
SIRONA S2 300 SENSOR SHEATHS UNIVERSAL SHEATHS SIZE 1 (300/PKG)
SIRONA DENTAL, INC.
Instrument/equipment drape, single-use, non-sterile| Primary Device ID | D764B10700181 |
| NIH Device Record Key | 08e3d2bd-2396-44a9-a01e-c90983830ed3 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | XIOS |
| Version Model Number | B1070018 |
| Company DUNS | 078613913 |
| Company Name | SIRONA DENTAL, INC. |
| Device Count | 300 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | (877) 724-4254 |
| customerserviceny@sirona.com | |
| Phone | (877) 724-4254 |
| customerserviceny@sirona.com | |
| Phone | (877) 724-4254 |
| customerserviceny@sirona.com | |
| Phone | (877) 724-4254 |
| customerserviceny@sirona.com | |
| Phone | (877) 724-4254 |
| customerserviceny@sirona.com | |
| Phone | (877) 724-4254 |
| customerserviceny@sirona.com | |
| Phone | (877) 724-4254 |
| customerserviceny@sirona.com | |
| Phone | (877) 724-4254 |
| customerserviceny@sirona.com | |
| Phone | (877) 724-4254 |
| customerserviceny@sirona.com | |
| Phone | (877) 724-4254 |
| customerserviceny@sirona.com | |
| Phone | (877) 724-4254 |
| customerserviceny@sirona.com | |
| Phone | (877) 724-4254 |
| customerserviceny@sirona.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | D764B10700180 [Unit of Use] |
| HIBCC | D764B10700181 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K963664
FDA Product Code
| EFB | Handpiece, Air-Powered, Dental |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2022-12-09 |
| Device Publish Date | 2016-09-09 |
On-Brand Devices [XIOS]
| D76461736241 | SIRONA HOLDER STARTER KIT S2 SHEATH |
| D76462018211 | SIRONA XIOS+ HOLDER STARTER KIT S2 SHEATH |
| D76462018131 | SIRONA XIOS+ HOLDER STARTER KIT S1 SHEATH |
| D76461765511 | SIRONA ENDO HLDRR ASSY KIT |
| D76461765441 | SIRONA ENDODONTIC TAB ASY KIT GREEN |
| D76461765361 | SIRONA BITEWING HLDR ASSY KIT RED |
| D76461765281 | SIRONA PERIAPICAL HLDR ASY KIT YELLOW |
| D76461765101 | SIRONA ANTERIOR HLDR ASY KIT BLUE |
| D76461736321 | SIRONA HOLDER STARTER KIT S1 SHEATH |
| D764B10700181 | SIRONA S2 300 SENSOR SHEATHS UNIVERSAL SHEATHS SIZE 1 (300/PKG) |
| D764B10700171 | SIRONA S1 300 SENSOR SHEATHS UNIVERSAL SHEATHS SIZE 1 (300/PKG) |
| D76461490041 | SIRONA XIOS+ SENSOR SHEATH SIZE 2 UNIVERSAL SHEATHS SIZE 1 (300/PKG) |
| D76461489981 | SIRONA XIOS+ SENSOR SHEATH SIZE 1 UNIVERSAL SHEATHS SIZE 1 (300/PKG) |
| D76461443771 | SIRONA USB 2 SHIPPING KIT |
| D76461443511 | USB A/B 3M BLACK CABLE W/FERRITE |
| D76461442861 | SIRONA PHX SENSOR SIZE 1 SHIP KIT |
| D76461442781 | SIRONA PHX SENSOR SIZE 2 SHIP KIT |
Trademark Results [XIOS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
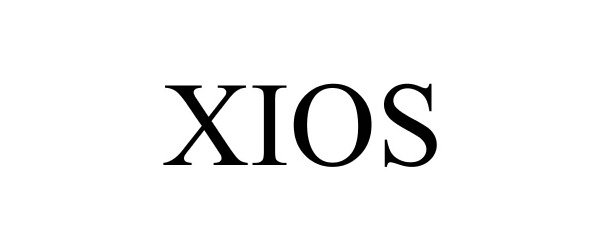 XIOS 87133269 5656213 Live/Registered |
DELL INC. 2016-08-10 |
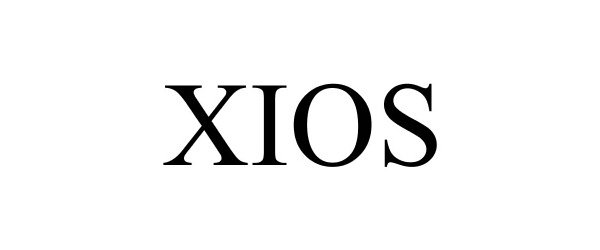 XIOS 85898237 4561202 Live/Registered |
Nikken, Inc. 2013-04-08 |
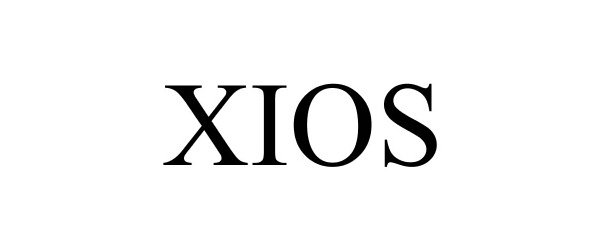 XIOS 85898228 4507775 Live/Registered |
Nikken, Inc. 2013-04-08 |
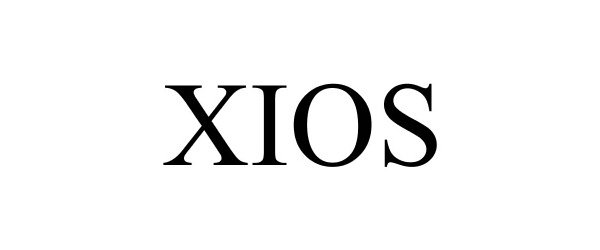 XIOS 85898212 not registered Dead/Abandoned |
Nikken, Inc. 2013-04-08 |
 XIOS 85693922 not registered Dead/Abandoned |
Draper/Hunt Development 2012-08-02 |
 XIOS 79069612 3811045 Dead/Cancelled |
Xios Qingdao Garment Co., Ltd 2009-02-16 |
 XIOS 79043028 3492890 Dead/Cancelled |
Sirona Dental Systems GmbH 2007-07-25 |
 XIOS 77299582 3699540 Live/Registered |
FLAHERTY, MICHAEL B. 2007-10-09 |
 XIOS 76531903 2940616 Live/Registered |
Park, Young Ho 2003-07-14 |
 XIOS 75807071 not registered Dead/Abandoned |
UniDial Communications, Inc. 1999-09-23 |
 XIOS 74393264 1907835 Live/Registered |
Flaherty, B. Michael 1993-05-21 |
 XIOS 74393263 1912441 Live/Registered |
Flaherty, B. Michael 1993-05-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.