Stage-1
GUDID D768S15014810K0
Tap
KEYSTONE DENTAL, INC.
Fixture/appliance dental drill bit, reusable| Primary Device ID | D768S15014810K0 |
| NIH Device Record Key | df08e193-097a-4015-93a9-dbe92c503807 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Stage-1 |
| Version Model Number | S1501-48-10K |
| Company DUNS | 787471015 |
| Company Name | KEYSTONE DENTAL, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(781)328-3313 |
| sshahid@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | D768S15014810K0 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K072768
FDA Product Code
| DZA | DRILL, DENTAL, INTRAORAL |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2020-01-09 |
| Device Publish Date | 2019-05-08 |
On-Brand Devices [Stage-1]
Trademark Results [Stage-1]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
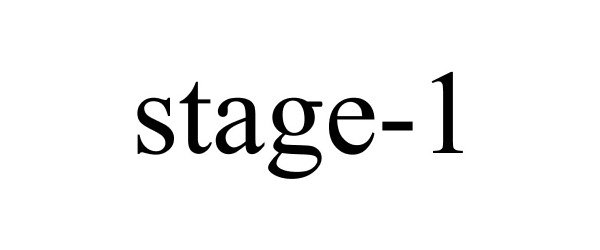 STAGE-1 87249703 5278164 Live/Registered |
U.S. Tsubaki Holdings, Inc. 2016-11-28 |
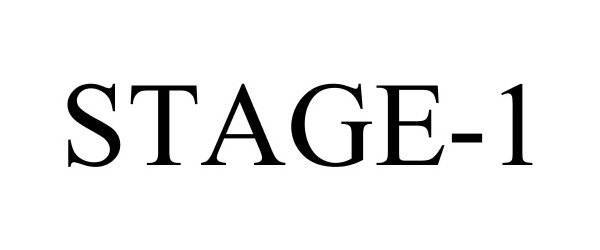 STAGE-1 78790766 not registered Dead/Abandoned |
Raytech Systems, Inc. 2006-01-12 |
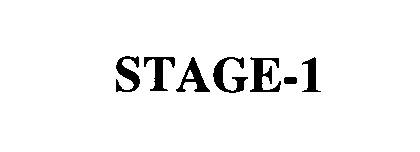 STAGE-1 76447176 2901128 Dead/Cancelled |
SBT NEWCO, INC. 2002-09-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.