Stage 1 S2501-70-1K
GUDID D768S2501701K0
COC Abutment
KEYSTONE DENTAL, INC.
Dental implant suprastructure, permanent, preformed| Primary Device ID | D768S2501701K0 |
| NIH Device Record Key | 0818de06-ad91-4b3a-b450-fd94d4fb8cc0 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Stage 1 |
| Version Model Number | S2501-70-1K |
| Catalog Number | S2501-70-1K |
| Company DUNS | 787471015 |
| Company Name | KEYSTONE DENTAL, INC. |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(781)328-3515 |
| cyoung@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com | |
| Phone | +1(866)902-9272 |
| info@keystonedental.com |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | D768S2501701K0 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K003226
FDA Product Code
| NHA | ABUTMENT, IMPLANT, DENTAL, ENDOSSEOUS |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | true |
[D768S2501701K0]
Radiation Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 5 |
| Public Version Date | 2019-11-08 |
| Device Publish Date | 2016-02-04 |
On-Brand Devices [Stage 1]
| D768S2504K0 | Preppable Abutment |
| D768S2501701K0 | COC Abutment |
| D768S2501551K0 | COC Abutment |
| D768S2501401K0 | COC Abutment |
| D768S2435K0 | Preppable Abutment |
| D768S2432701K0 | COC Abutment |
| D768S2432551K0 | COC Abutment |
| D768S2432401K0 | COC Abutment |
| D768S24172K0 | Oring Abutment |
| D768S24170K0 | Oring Abutment |
| D768S2408K0 | Abutment Screw |
| D768S240501K0 | Gold Sleeve |
| D768S240500K0 | Gold Sleeve |
| D768S2400K0 | Index Abutment |
| D76842020K0 | Snap Abutment |
| D76842019K0 | Snap Abutment |
| D76842018K0 | Snap Abutment |
| D76842017K0 | Snap Abutment |
| D76842014K0 | Abutment Screw |
| D76842008K0 | Gold Sleeve |
| D76842007K0 | Gold Sleeve |
| D76842004K0 | Index Abutment |
| D76842002K0 | Angled Abutment |
| D76842001K | Angled Abutment |
| D76842000K0 | Abutment Screw |
| D768S2506K0 | Healing Cap |
| D768S2439K0 | Healing Cap |
| D768S243570K0 | Quick Cap |
| D768S240701K0 | Temp Sleeve |
| D768S240700K0 | Temp Sleeve |
| D768S2401K0 | Healing Cap |
| D768S180645K0 | Cover Screw |
| D768S180630K0 | Cover Screw |
| D768S180045K0 | Cover Screw |
| D768S180030K0 | Cover Screw |
| D768S180015K0 | Cover Screw |
| D768S180000K0 | Cover Screw |
| D768S1800000 | Cover Screw |
| D76842026K0 | Healing Cap |
| D76842025K0 | Healing Cap |
| D76842024K0 | Healing Cap |
| D76842012K0 | Temp Sleeve |
| D76842011K0 | Temp Sleeve |
| D76842005K0 | Healing Cap |
Trademark Results [Stage 1]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 STAGE 1 97912993 not registered Live/Pending |
Photon Effect, LLC 2023-04-28 |
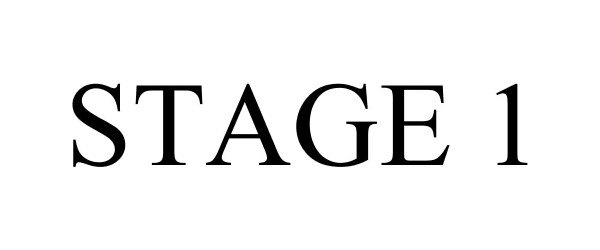 STAGE 1 87586885 not registered Dead/Abandoned |
DATA CENTER STANDARDS FOUNDATION 2017-08-28 |
 STAGE 1 87586851 not registered Dead/Abandoned |
DATA CENTER STANDARDS FOUNDATION 2017-08-28 |
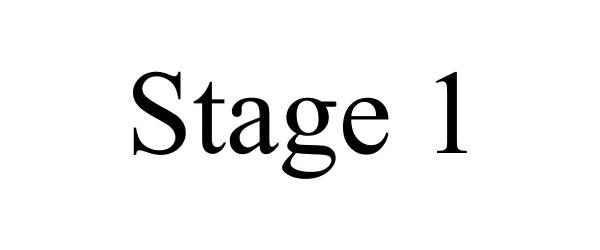 STAGE 1 85196960 4218515 Dead/Cancelled |
Coast to Coast Entertainment, LLC 2010-12-13 |
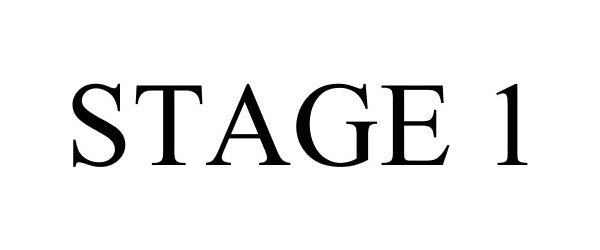 STAGE 1 77282042 not registered Dead/Abandoned |
Vayser, Felix 2007-09-18 |
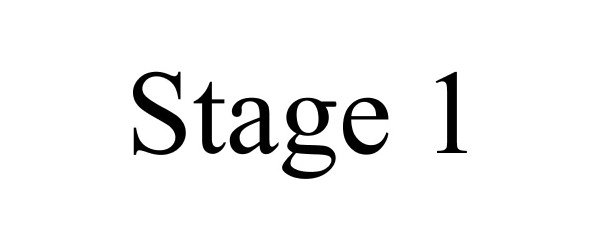 STAGE 1 77061246 not registered Dead/Abandoned |
Saga Musical Instruments 2006-12-11 |
 STAGE 1 76511268 not registered Dead/Abandoned |
Pola Chemical Industries Inc. 2003-05-01 |
 STAGE 1 74294826 1756410 Live/Registered |
BEECH-NUT NUTRITION CORPORATION 1992-07-16 |
 STAGE 1 74129173 not registered Dead/Abandoned |
Ralston Purina Company 1991-01-09 |
 STAGE 1 73087983 1059088 Dead/Cancelled |
Walter Kidde & Co., Inc. 1976-05-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.