MYSTIQUE® MB K110-532-10
GUDID D807K110532101
MYSTIQUE® MB RT RX 022 U 5-5/L3-3 CS HK
GAC
Orthodontic bracket, ceramic| Primary Device ID | D807K110532101 |
| NIH Device Record Key | c0cabd9c-c322-45fd-89e9-da37d1d802d7 |
| Commercial Distribution Discontinuation | 2020-01-31 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | MYSTIQUE® MB |
| Version Model Number | K110-532-10 |
| Catalog Number | K110-532-10 |
| Company DUNS | 053475903 |
| Company Name | GAC |
| Device Count | 16 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | D807K110532100 [Unit of Use] |
| HIBCC | D807K110532101 [Primary] |
FDA Product Code
| NJM | Bracket, ceramic, orthodontic |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2020-01-31 |
| Device Publish Date | 2017-06-26 |
On-Brand Devices [MYSTIQUE® MB]
| D807K110532101 | MYSTIQUE® MB RT RX 022 U 5-5/L3-3 CS HK |
| D807K110531111 | MYSTIQUE® MB RT RX 018 U 5-5/L3-3 CS-BC HK |
| D807K110531101 | MYSTIQUE® MB RT RX 018 U5-5/L3-3 CS HK |
| D807K110152101 | MYSTIQUE® MB RT RX 022 U 5-5 CS HK |
| D807K110151101 | MYSTIQUE® MB RT RX 018 U 5-5 CS HK |
Trademark Results [MYSTIQUE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MYSTIQUE 98740195 not registered Live/Pending |
Mystique IP LLC 2024-09-09 |
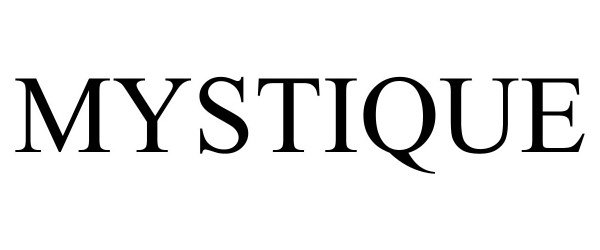 MYSTIQUE 98629310 not registered Live/Pending |
Company 19 LLC 2024-07-02 |
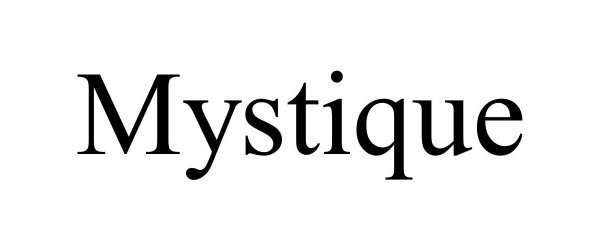 MYSTIQUE 98608736 not registered Live/Pending |
Sheikh Faroozan 2024-06-19 |
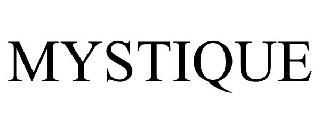 MYSTIQUE 98555178 not registered Live/Pending |
Focarino, Diane 2024-05-16 |
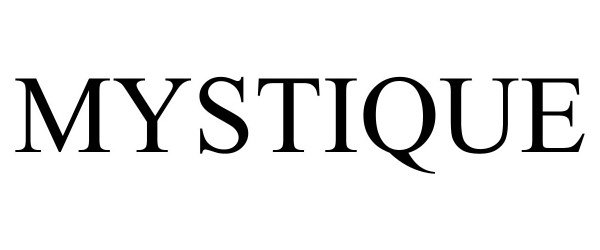 MYSTIQUE 98530638 not registered Live/Pending |
Mystique at Waterpark Place Condominium Association, Inc. 2024-05-02 |
 MYSTIQUE 97483434 not registered Live/Pending |
Gillespie, Angela 2022-06-30 |
 MYSTIQUE 90688809 not registered Live/Pending |
Mystique Boatworks, LLC 2021-05-04 |
 MYSTIQUE 88799990 not registered Live/Pending |
Ambiance Fireplaces LLC 2020-02-17 |
 MYSTIQUE 88332086 not registered Live/Pending |
San Fang Chemical Industry Co., Ltd. 2019-03-08 |
 MYSTIQUE 87959783 5837500 Live/Registered |
EATDRINKNJOY GIDA ITHALAT IHRACAT SANAYI VE TICARET LIMITED SIRKETI 2018-06-13 |
 MYSTIQUE 87653114 5944715 Live/Registered |
Big Night Entertainment Group, Inc. 2017-10-20 |
 MYSTIQUE 87582213 not registered Live/Pending |
Pelican 1 Owner LLC 2017-08-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.