Coil
GUDID D972AMC215COAX300
0215 COAX 30'/#3 BOBBIN
Acme Monaco Corporation
Orthodontic archwire| Primary Device ID | D972AMC215COAX300 |
| NIH Device Record Key | 7dfb2946-dfae-43f4-bc0e-2b4c7be01f2b |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Coil |
| Version Model Number | AMC215COAX30 |
| Company DUNS | 118370238 |
| Company Name | Acme Monaco Corporation |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | false |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 8602241349 |
| acmecorp@acmemonaco.com | |
| Phone | 8602241349 |
| acmecorp@acmemonaco.com | |
| Phone | 8602241349 |
| acmecorp@acmemonaco.com | |
| Phone | 8602241349 |
| acmecorp@acmemonaco.com | |
| Phone | 8602241349 |
| acmecorp@acmemonaco.com | |
| Phone | 8602241349 |
| acmecorp@acmemonaco.com | |
| Phone | 8602241349 |
| acmecorp@acmemonaco.com | |
| Phone | 8602241349 |
| acmecorp@acmemonaco.com | |
| Phone | 8602241349 |
| acmecorp@acmemonaco.com | |
| Phone | 8602241349 |
| acmecorp@acmemonaco.com | |
| Phone | 8602241349 |
| acmecorp@acmemonaco.com | |
| Phone | 8602241349 |
| acmecorp@acmemonaco.com | |
| Phone | 8602241349 |
| acmecorp@acmemonaco.com | |
| Phone | 8602241349 |
| acmecorp@acmemonaco.com | |
| Phone | 8602241349 |
| acmecorp@acmemonaco.com | |
| Phone | 8602241349 |
| acmecorp@acmemonaco.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | D972AMC215COAX300 [Primary] |
FDA Product Code
| DZC | Wire, Orthodontic |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-08-09 |
| Device Publish Date | 2024-08-01 |
On-Brand Devices [Coil]
| D972AMC10X30X36C2 | SS Closed Coil10x30 / 91 cm |
| D972AMC10X30X362 | SS Open coil 10x30 /91 cm |
| D972AMC10309MME2 | NiTi Closed Eyelet Spring - 9 mm - 50 pcs |
| D972AMC103021OPN2 | NiTi Open Coil 10x30 |
| D972AMC123021OPN0 | 12X30 OPEN NITI SPRING 21"/#3 BOBBIN |
| D972AMC10X30X36C0 | UDI World Biotech UDI |
| D972AMC215COAX300 | 0215 COAX 30'/#3 BOBBIN |
Trademark Results [Coil]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
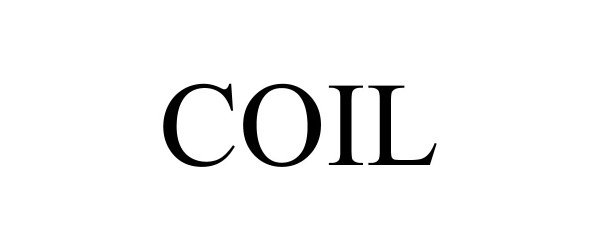 COIL 90456228 not registered Live/Pending |
Chris Phelps Wines LLC 2021-01-08 |
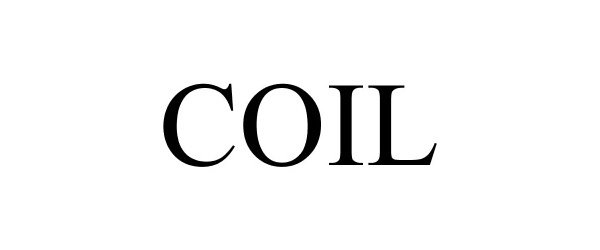 COIL 88402744 not registered Live/Pending |
Rthm Technologies Inc. 2019-04-25 |
 COIL 88306417 not registered Live/Pending |
Rthm Technologies Inc. 2019-02-19 |
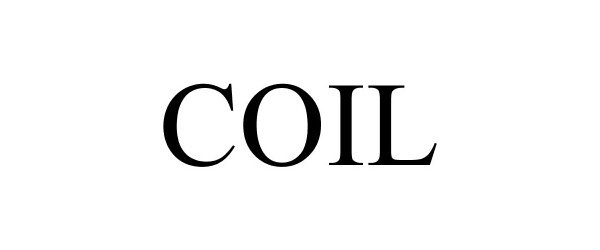 COIL 88306411 not registered Live/Pending |
Rthm Technologies Inc. 2019-02-19 |
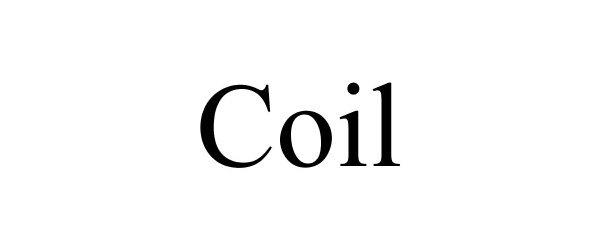 COIL 88268670 not registered Dead/Abandoned |
Penn, Bobby 2019-01-20 |
 COIL 88242642 not registered Live/Pending |
Coil Technologies, Inc. 2018-12-27 |
 COIL 88242639 not registered Live/Pending |
Coil Technologies, Inc. 2018-12-27 |
 COIL 88242637 not registered Live/Pending |
Coil Technologies, Inc. 2018-12-27 |
 COIL 87449838 5364035 Live/Registered |
The State University of New York 2017-05-15 |
 COIL 87079990 not registered Dead/Abandoned |
CoiL 2016-06-22 |
 COIL 86587507 not registered Dead/Abandoned |
Monika Pearson 2015-04-04 |
 COIL 86482119 4910274 Live/Registered |
Misc. Goods Co., LLC 2014-12-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.