declipse®SPECT SE 1301-0002
GUDID ESURSE13010002
SurgicEye GmbH
Scintillation radiation measuring probe| Primary Device ID | ESURSE13010002 |
| NIH Device Record Key | 823cf8c0-460a-464e-86a9-f71f32cae389 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | declipse®SPECT |
| Version Model Number | declipseSPECT Viewer |
| Catalog Number | SE 1301-0002 |
| Company DUNS | 340279325 |
| Company Name | SurgicEye GmbH |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +4989549989050 |
| support@surgiceye.com |
Operating and Storage Conditions
| Handling Environment Atmospheric Pressure | Between 70 KiloPascal and 106 KiloPascal |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | ESURSE13010002 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K123917
FDA Product Code
| IZD | Probe, Uptake, Nuclear |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2018-01-17 |
Trademark Results [declipse]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
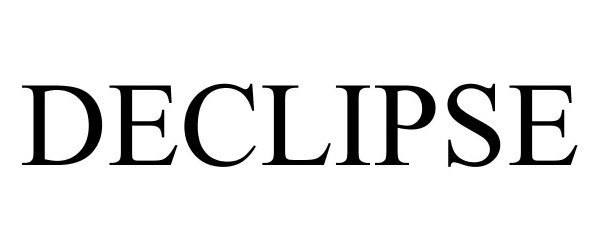 DECLIPSE 88167274 not registered Live/Pending |
Margaret A. Moore 2018-10-24 |
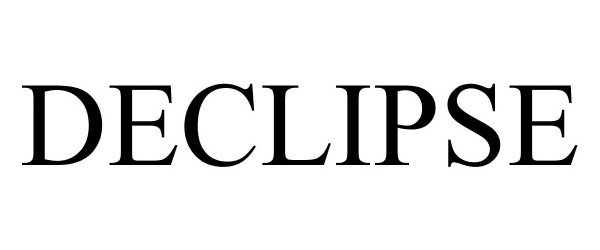 DECLIPSE 87681819 not registered Live/Pending |
Moore, Margaret A. 2017-11-13 |
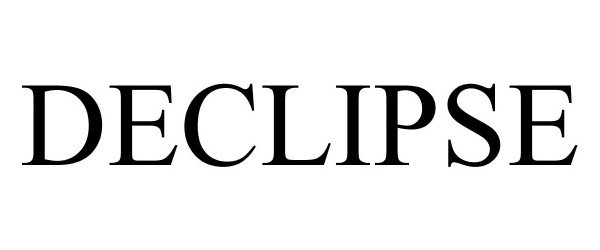 DECLIPSE 87497844 not registered Live/Pending |
Moore, Margaret A. 2017-06-20 |
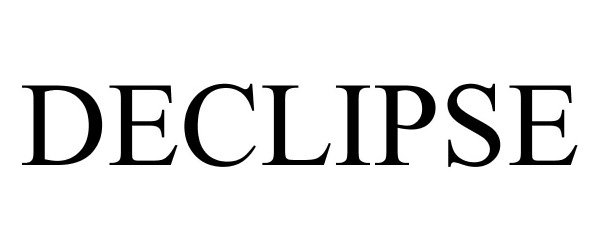 DECLIPSE 85227591 4386182 Live/Registered |
SurgicEye GmbH 2011-01-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.