Venus Diamond 66081848
GUDID J014660818480
Venus Diamond Syringe Sample Kit ONE
Heraeus Kulzer GmbH
Dental composite resin| Primary Device ID | J014660818480 |
| NIH Device Record Key | e5583f80-7bd8-4dde-be5d-255a3a9bd38d |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Venus Diamond |
| Version Model Number | 66081848 |
| Catalog Number | 66081848 |
| Company DUNS | 315666321 |
| Company Name | Heraeus Kulzer GmbH |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx | |
| Phone | +1(800)431-1785 |
| xx@xx.xx |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | J014660818480 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K073554
FDA Product Code
| EBF | MATERIAL, TOOTH SHADE, RESIN |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-03-30 |
| Device Publish Date | 2020-03-20 |
On-Brand Devices [Venus Diamond]
| J014660818490 | Venus Diamond PLT Sample Kit ONE |
| J014660818480 | Venus Diamond Syringe Sample Kit ONE |
| J014660818430 | Venus Diamond ONE Kit-PLT |
| J014660818420 | Venus Diamond ONE Kit-Syringe |
| J014660818391 | Venus Diamond PLT Refill 20x0.25G ONE |
| J014660818381 | Venus Diamond Syring Refill 1X4G ONE |
| J014660968750 | VENUS DIAMOND SYRINGE REFILL 1x4G BLEACH |
| J014660968740 | VENUS DIAMOND SYRINGE REFILL 1x4G DARK |
| J014660968730 | VENUS DIAMOND SYRINGE REFILL 1x4G MEDIUM |
| J014660968720 | VENUS DIAMOND SYRINGE REFILL 1x4G LIGHT |
| J014660968710 | VENUS DIAMOND PLT REFILL, 20x0, 25G BLEACH |
| J014660968700 | VENUS DIAMOND PLT REFILL, 20x0, 25G DARK |
| J014660968690 | VENUS DIAMOND PLT REFILL, 20x0, 25G MEDIUM |
| J014660968680 | VENUS DIAMOND PLT REFILL, 20x0, 25G LIGHT |
| J014660982650 | VENUS DIAMOND PURE KIT - SYRINGE |
| J014660982640 | VENUS DIAMOND PURE KIT - PLT |
| J014660982610 | VENUS DIAMOND SYRINGE SAMPLE KIT MEDIUM |
| J014660982600 | VENUS DIAMOND PLT SAMPLE KIT MEDIUM |
| J014660982770 | PROC Venus Diamond Kit 2x4G SYR |
| J014660982750 | PROC Venus Diamond Kit 2x20X0.25G PLT |
| J014660390230 | VENUS DIAMOND PLT REFILL 2x10x0,25G OD |
| J014660390220 | VENUS DIAMOND PLT REFILL 2x10x0,25G OM |
| J014660390210 | VENUS DIAMOND PLT REFILL 1x10x0,25G OL |
| J014660354860 | VENUS DIAMOND SYRINGE REFILL 1x4G OD |
| J014660354850 | VENUS DIAMOND SYRINGE REFILL 1x4G OM |
| J014660354840 | VENUS DIAMOND SYRINGE REFILL 1x4G OL |
Trademark Results [Venus Diamond]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
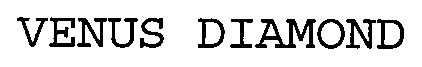 VENUS DIAMOND 76693930 3656259 Live/Registered |
KULZER GMBH 2008-10-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.