Fixate™ Suturing Device FB-201-01
GUDID M365FB201010
Fixate™ Suturing Device
BOSTON SCIENTIFIC NEUROMODULATION CORPORATION
Analgesic spinal cord electrical stimulation system| Primary Device ID | M365FB201010 |
| NIH Device Record Key | 8d583171-e61c-4412-b94f-fbc16b9db58f |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Fixate™ Suturing Device |
| Version Model Number | FB-201-01 |
| Catalog Number | FB-201-01 |
| Company DUNS | 824951958 |
| Company Name | BOSTON SCIENTIFIC NEUROMODULATION CORPORATION |
| Device Count | 1 |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | M365FB201010 [Primary] |
FDA Product Code
| GZB | STIMULATOR, SPINAL-CORD, IMPLANTED (PAIN RELIEF) |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | true |
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
[M365FB201010]
Ethylene Oxide
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2021-02-05 |
| Device Publish Date | 2014-09-24 |
On-Brand Devices [Fixate™ Suturing Device]
| M365FB201010 | Fixate™ Suturing Device |
| M365FB101010 | Fixate™ Suturing Device |
Trademark Results [Fixate]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
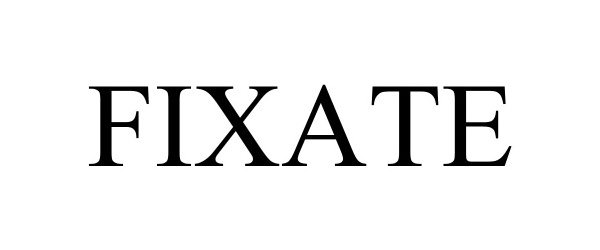 FIXATE 98009420 not registered Live/Pending |
Boston Scientific Neuromodulation Corporation 2023-05-23 |
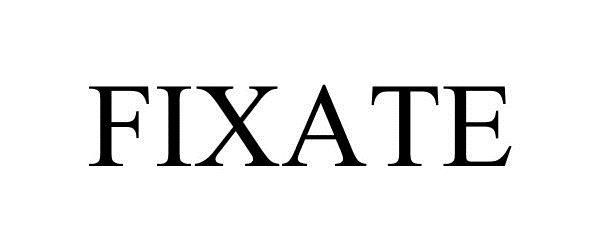 FIXATE 87975114 5183631 Live/Registered |
Beachbody, LLC 2016-06-15 |
 FIXATE 87214719 not registered Dead/Abandoned |
152310 Canada 2016-10-25 |
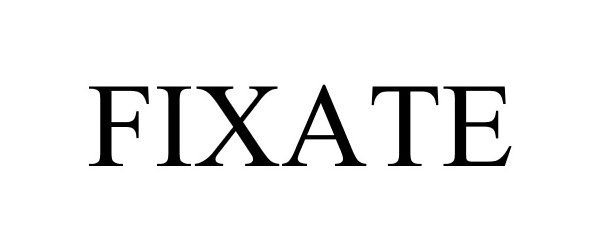 FIXATE 87071993 not registered Dead/Abandoned |
Beachbody, LLC 2016-06-15 |
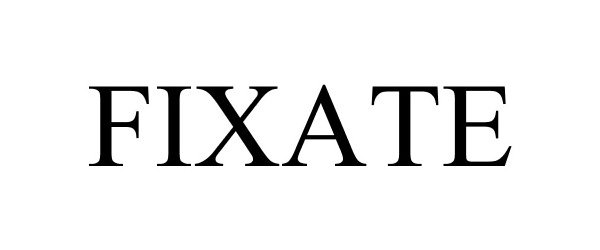 FIXATE 87034517 5173069 Live/Registered |
FIXATE, INC. 2016-05-12 |
 FIXATE 86585481 4848055 Live/Registered |
Beachbody, LLC 2015-04-02 |
 FIXATE 86550668 not registered Dead/Abandoned |
LFS Products, LLC 2015-03-02 |
 FIXATE 85903736 4523247 Live/Registered |
Farkas, Kevin 2013-04-14 |
 FIXATE 85482334 4175586 Live/Registered |
CHS INC. 2011-11-29 |
 FIXATE 85324146 not registered Dead/Abandoned |
QUALCOMM Incorporated 2011-05-18 |
 FIXATE 79399130 not registered Live/Pending |
SnackHQ Pty Ltd 2024-04-23 |
 FIXATE 76441458 not registered Dead/Abandoned |
RUTLEDGE, MICHAEL J. 2002-08-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.