X-Box 8881422
GUDID M71188814220
PEEK, Ta
Innovasis, Inc.
Polymeric spinal interbody fusion cage| Primary Device ID | M71188814220 |
| NIH Device Record Key | b0b8769d-f210-4b59-8988-180b95e17e99 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | X-Box |
| Version Model Number | 8881422 |
| Catalog Number | 8881422 |
| Company DUNS | 156567492 |
| Company Name | Innovasis, Inc. |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com |
Device Dimensions
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
| Length | 22 Millimeter |
| Height | 14 Millimeter |
| Width | 8 Millimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | M71188814220 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K062151
FDA Product Code
| MQP | Spinal Vertebral Body Replacement Device |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
[M71188814220]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2020-02-24 |
| Device Publish Date | 2015-01-22 |
On-Brand Devices [X-Box]
| M711LCI010 | SST |
| M71190814250 | PEEK, Ta |
| M71190812250 | PEEK, Ta |
| M71190810250 | PEEK, Ta |
| M71190808250 | PEEK, Ta |
| M71188814250 | PEEK, Ta |
| M71188814220 | PEEK, Ta |
| M71188812250 | PEEK, Ta |
| M71188812220 | PEEK, Ta |
| M71188810250 | PEEK, Ta |
| M71188810220 | PEEK, Ta |
| M71188808250 | PEEK, Ta |
| M71188808220 | PEEK, Ta |
| M71188016250 | PEEK, Ta |
| M71188014250 | PEEK, Ta |
| M71188012250 | PEEK, Ta |
| M71188010250 | PEEK, Ta |
| M71188008250 | PEEK, Ta |
| M71181014220 | PEEK, Ta |
| M71181012220 | PEEK, Ta |
| M71181010220 | PEEK, Ta |
| M71181008220 | PEEK, Ta |
| M71180814220 | PEEK, Ta |
| M71180812220 | PEEK, Ta |
| M71180810220 | PEEK, Ta |
| M71180808220 | PEEK, Ta |
| M711LCR010 | SST |
| M711BTA690 | Polypropylene, Radel, SST |
| M711BTA680 | Polypropylene, Radel, SST |
| M711BTA670 | Al, SST, Nylon, Silicone |
| M711LCS140 | SST |
| M711LCS130 | SST |
| M711LCS120 | SST |
| M711LCS110 | SST |
| M711LCS100 | SST |
| M711LCS090 | SST |
| M711LCS080 | SST |
| M711LCS070 | SST |
| M711LCD140 | SST |
| M711LCD130 | SST |
| M711LCD120 | SST |
| M711LCD110 | SST |
| M711LCD100 | SST |
| M711LCD090 | SST |
| M711LCD080 | SST |
| M711LCD070 | SST |
Trademark Results [X-Box]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
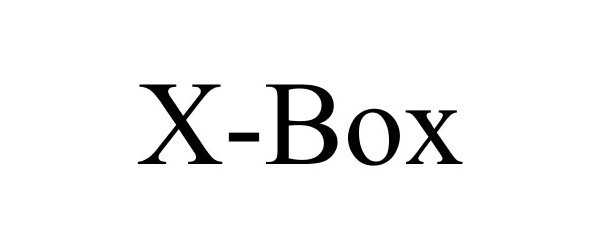 X-BOX 78877684 not registered Dead/Abandoned |
Innovasis 2006-05-05 |
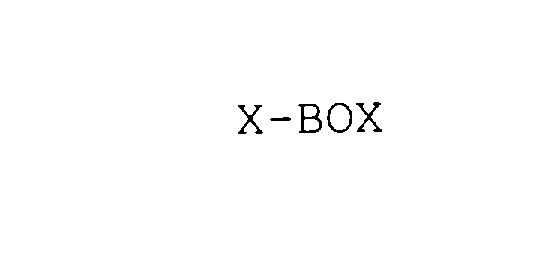 X-BOX 78000501 not registered Dead/Abandoned |
Risner, Jerry, L 2000-03-22 |
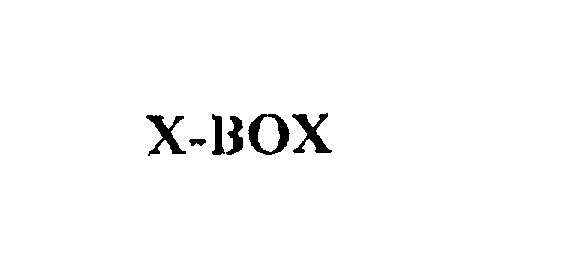 X-BOX 75829146 not registered Dead/Abandoned |
Microsoft Corporation 1999-10-18 |
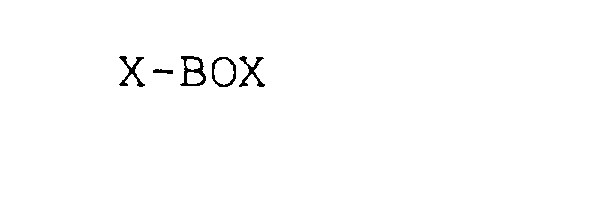 X-BOX 75820514 not registered Dead/Abandoned |
CD-3D Corporation 1999-11-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.