LxHA™ LS-506-10
GUDID M711LS506100
Ti 6Al 4V (ELI)
INNO Holdings, Inc.
Spinal implant trial| Primary Device ID | M711LS506100 |
| NIH Device Record Key | 4b5a4ebd-877c-4c47-89e2-bb134db4aba2 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | LxHA™ |
| Version Model Number | LS-506-10 |
| Catalog Number | LS-506-10 |
| Company DUNS | 141620464 |
| Company Name | INNO Holdings, Inc. |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com | |
| Phone | 801-261-2236 |
| info@innovasis.com |
Device Dimensions
| Width | 26 Millimeter |
| Height | 16 Millimeter |
| Angle | 8 degree |
| Width | 26 Millimeter |
| Height | 16 Millimeter |
| Angle | 8 degree |
| Width | 26 Millimeter |
| Height | 16 Millimeter |
| Angle | 8 degree |
| Width | 26 Millimeter |
| Height | 16 Millimeter |
| Angle | 8 degree |
| Width | 26 Millimeter |
| Height | 16 Millimeter |
| Angle | 8 degree |
| Width | 26 Millimeter |
| Height | 16 Millimeter |
| Angle | 8 degree |
| Width | 26 Millimeter |
| Height | 16 Millimeter |
| Angle | 8 degree |
| Width | 26 Millimeter |
| Height | 16 Millimeter |
| Angle | 8 degree |
| Width | 26 Millimeter |
| Height | 16 Millimeter |
| Angle | 8 degree |
| Width | 26 Millimeter |
| Height | 16 Millimeter |
| Angle | 8 degree |
| Width | 26 Millimeter |
| Height | 16 Millimeter |
| Angle | 8 degree |
| Width | 26 Millimeter |
| Height | 16 Millimeter |
| Angle | 8 degree |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | M711LS506100 [Primary] |
FDA Product Code
| MAX | Intervertebral Fusion Device With Bone Graft, Lumbar |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[M711LS506100]
Moist Heat or Steam Sterilization
[M711LS506100]
Moist Heat or Steam Sterilization
[M711LS506100]
Moist Heat or Steam Sterilization
[M711LS506100]
Moist Heat or Steam Sterilization
[M711LS506100]
Moist Heat or Steam Sterilization
[M711LS506100]
Moist Heat or Steam Sterilization
[M711LS506100]
Moist Heat or Steam Sterilization
[M711LS506100]
Moist Heat or Steam Sterilization
[M711LS506100]
Moist Heat or Steam Sterilization
[M711LS506100]
Moist Heat or Steam Sterilization
[M711LS506100]
Moist Heat or Steam Sterilization
[M711LS506100]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2025-12-17 |
| Device Publish Date | 2019-07-31 |
On-Brand Devices [LxHA™]
| M711LXT221235081 | HA PEEK, Ta |
| M711LXL261660001 | HA PEEK, Ta |
| M711LXL181250161 | HA PEEK, Ta |
| M711LXL181045121 | HA PEEK, Ta |
| M711LS5070 | SST, Silicone, Al, Adhesive |
| M711LS506180 | Ti 6Al 4V (ELI) |
| M711LS506170 | Ti 6Al 4V (ELI) |
| M711LS506160 | Ti 6Al 4V (ELI) |
| M711LS506150 | Ti 6Al 4V (ELI) |
| M711LS506140 | Ti 6Al 4V (ELI) |
| M711LS506130 | Ti 6Al 4V (ELI) |
| M711LS506120 | Ti 6Al 4V (ELI) |
| M711LS506110 | Ti 6Al 4V (ELI) |
| M711LS506100 | Ti 6Al 4V (ELI) |
| M711LS506090 | Ti 6Al 4V (ELI) |
| M711LS506080 | Ti 6Al 4V (ELI) |
| M711LS506070 | Ti 6Al 4V (ELI) |
| M711LS506060 | Ti 6Al 4V (ELI) |
| M711LS506050 | Ti 6Al 4V (ELI) |
| M711LS506040 | Ti 6Al 4V (ELI) |
| M711LS506030 | Ti 6Al 4V (ELI) |
| M711LS506020 | Ti 6Al 4V (ELI) |
| M711LS506010 | Ti 6Al 4V (ELI) |
| M711LS505200 | Ti 6Al 4V (ELI) |
| M711LS505190 | Ti 6Al 4V (ELI) |
| M711LS505180 | Ti 6Al 4V (ELI) |
| M711LS505170 | Ti 6Al 4V (ELI) |
| M711LS505160 | Ti 6Al 4V (ELI) |
| M711LS505150 | Ti 6Al 4V (ELI) |
| M711LS505140 | Ti 6Al 4V (ELI) |
| M711LS505130 | Ti 6Al 4V (ELI) |
| M711LS505120 | Ti 6Al 4V (ELI) |
| M711LS505110 | Ti 6Al 4V (ELI) |
| M711LS505100 | Ti 6Al 4V (ELI) |
| M711LS505090 | Ti 6Al 4V (ELI) |
| M711LS505080 | Ti 6Al 4V (ELI) |
| M711LS505070 | Ti 6Al 4V (ELI) |
| M711LS505060 | Ti 6Al 4V (ELI) |
| M711LS505050 | Ti 6Al 4V (ELI) |
| M711LS505040 | Ti 6Al 4V (ELI) |
| M711LS505030 | Ti 6Al 4V (ELI) |
| M711LS505020 | Ti 6Al 4V (ELI) |
| M711LS505010 | Ti 6Al 4V (ELI) |
| M711LS504210 | Ti 6Al 4V (ELI) |
| M711LS504200 | Ti 6Al 4V (ELI) |
| M711LS504190 | Ti 6Al 4V (ELI) |
| M711LS504180 | Ti 6Al 4V (ELI) |
| M711LS504170 | Ti 6Al 4V (ELI) |
| M711LS504160 | Ti 6Al 4V (ELI) |
| M711LS504150 | Ti 6Al 4V (ELI) |
Trademark Results [LxHA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
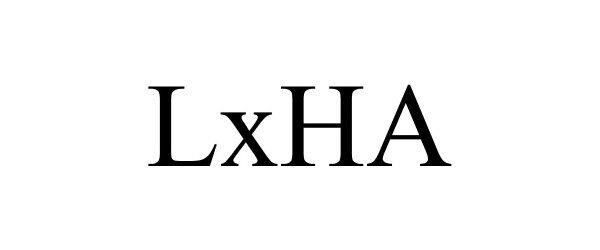 LXHA 88127521 not registered Live/Pending |
Innovasis, Inc. 2018-09-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.