Cimeosil® Scar Management, Gel Sheet GS-1215T
GUDID M724GS1215T1
Scar Management, Gel Sheet
IMPLANTECH ASSOCIATES, INC.
Scar-management dressing, reusable| Primary Device ID | M724GS1215T1 |
| NIH Device Record Key | 40af8d02-99ca-460c-8fc9-ff6c844a7921 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Cimeosil® Scar Management, Gel Sheet |
| Version Model Number | 12cm x 15cm |
| Catalog Number | GS-1215T |
| Company DUNS | 784664955 |
| Company Name | IMPLANTECH ASSOCIATES, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | 1-800-733-0833 |
| info@implantech.com | |
| Phone | 1-800-733-0833 |
| info@implantech.com | |
| Phone | 1-800-733-0833 |
| info@implantech.com | |
| Phone | 1-800-733-0833 |
| info@implantech.com | |
| Phone | 1-800-733-0833 |
| info@implantech.com | |
| Phone | 1-800-733-0833 |
| info@implantech.com | |
| Phone | 1-800-733-0833 |
| info@implantech.com | |
| Phone | 1-800-733-0833 |
| info@implantech.com | |
| Phone | 1-800-733-0833 |
| info@implantech.com | |
| Phone | 1-800-733-0833 |
| info@implantech.com | |
| Phone | 1-800-733-0833 |
| info@implantech.com | |
| Phone | 1-800-733-0833 |
| info@implantech.com | |
| Phone | 1-800-733-0833 |
| info@implantech.com |
Operating and Storage Conditions
| Storage Environment Temperature | Between 16 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 60 Degrees Fahrenheit and 85 Degrees Fahrenheit |
| Storage Environment Temperature | Between 16 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 60 Degrees Fahrenheit and 85 Degrees Fahrenheit |
| Storage Environment Temperature | Between 16 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 60 Degrees Fahrenheit and 85 Degrees Fahrenheit |
| Storage Environment Temperature | Between 16 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 60 Degrees Fahrenheit and 85 Degrees Fahrenheit |
| Storage Environment Temperature | Between 16 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 60 Degrees Fahrenheit and 85 Degrees Fahrenheit |
| Storage Environment Temperature | Between 16 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 60 Degrees Fahrenheit and 85 Degrees Fahrenheit |
| Storage Environment Temperature | Between 16 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 60 Degrees Fahrenheit and 85 Degrees Fahrenheit |
| Storage Environment Temperature | Between 16 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 60 Degrees Fahrenheit and 85 Degrees Fahrenheit |
| Storage Environment Temperature | Between 16 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 60 Degrees Fahrenheit and 85 Degrees Fahrenheit |
| Storage Environment Temperature | Between 16 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 60 Degrees Fahrenheit and 85 Degrees Fahrenheit |
| Storage Environment Temperature | Between 16 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 60 Degrees Fahrenheit and 85 Degrees Fahrenheit |
| Storage Environment Temperature | Between 16 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 60 Degrees Fahrenheit and 85 Degrees Fahrenheit |
| Storage Environment Temperature | Between 16 Degrees Celsius and 29 Degrees Celsius |
| Storage Environment Temperature | Between 60 Degrees Fahrenheit and 85 Degrees Fahrenheit |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | M724GS1215T1 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K982051
FDA Product Code
| MDA | Elastomer, Silicone, For Scar Management |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2024-02-16 |
| Device Publish Date | 2020-05-27 |
On-Brand Devices [Cimeosil® Scar Management, Gel Sheet]
| M724GSVM1 | Scar Management, Gel Sheet |
| M724GSLS1 | Scar Management, Gel Sheet |
| M724GSBR1 | Scar Management, Gel Sheet |
| M724GSAC1 | Scar Management, Gel Sheet |
| M724GS18X20T1 | Scar Management, Gel Sheet |
| M724GS18X025T1 | Scar Management, Gel Sheet |
| M724GS18X0251 | Scar Management, Gel Sheet |
| M724GS1215T1 | Scar Management, Gel Sheet |
| M724GS12151 | Scar Management, Gel Sheet |
| M724GS10121 | Scar Management, Gel Sheet |
| M724GS0610T1 | Scar Management, Gel Sheet |
| M724GS06101 | Scar and Laser Gel |
| M724GS04051 | Scar Management, Gel Sheet |
Trademark Results [Cimeosil]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
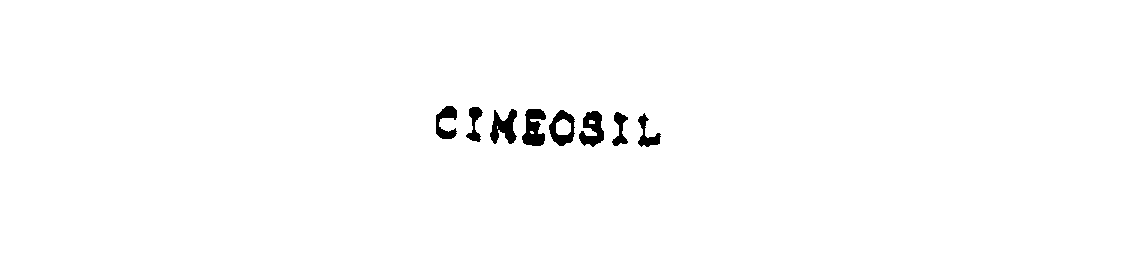 CIMEOSIL 76074984 2724924 Live/Registered |
IMPLANTECH ASSOCIATES, INC. 2000-06-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.