MAXUM
GUDID M737M53031
MAXUM CUSTOMIZATION SET BEADS
Ototronix, LLC
Partially-implantable middle ear implant system| Primary Device ID | M737M53031 |
| NIH Device Record Key | 127792fc-59fe-45ec-8262-73ca2e2eb41a |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | MAXUM |
| Version Model Number | M5303 |
| Company DUNS | 833067478 |
| Company Name | Ototronix, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | M737M53031 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Approval: P010023
FDA Product Code
| MPV | Implant, Hearing, Active, Middle Ear, Partially Implanted |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-04-06 |
| Device Publish Date | 2022-03-29 |
On-Brand Devices [MAXUM]
| M737M53031 | MAXUM CUSTOMIZATION SET BEADS |
| M737M31991 | MAXUM IPC FIT DEMO |
| M737M31101 | MAXUM IPC REMAKE |
Trademark Results [MAXUM]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
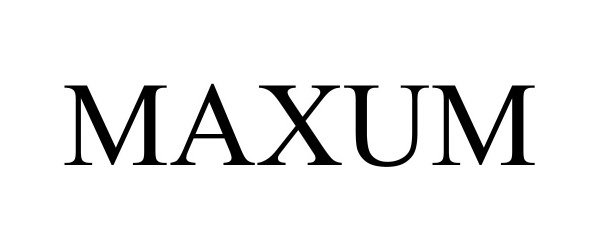 MAXUM 98500024 not registered Live/Pending |
MAXUM Fitness Inc. 2024-04-15 |
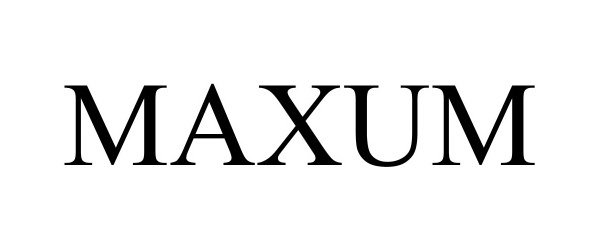 MAXUM 97756676 not registered Live/Pending |
Aftermarket Solutions LLC 2023-01-17 |
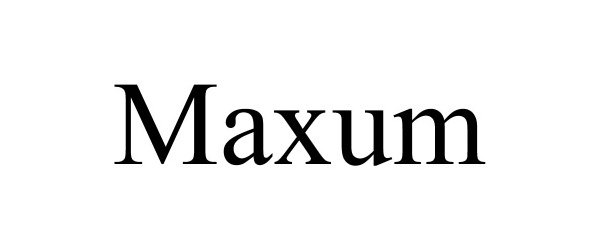 MAXUM 97137891 not registered Live/Pending |
Maxum Inc. 2021-11-22 |
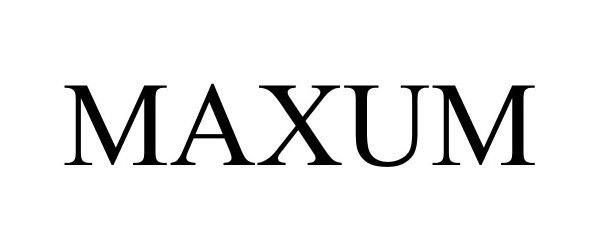 MAXUM 88202130 not registered Live/Pending |
Aftermarket Solutions LLC 2018-11-21 |
 MAXUM 87535658 not registered Dead/Abandoned |
Masco Cabinetry LLC 2017-07-20 |
 MAXUM 87413562 not registered Indifferent |
Masco Cabinetry LLC 0000-00-00 |
 MAXUM 87235617 not registered Dead/Abandoned |
Aftermarket Solutions LLC 2016-11-14 |
 MAXUM 86650803 4885341 Live/Registered |
MAXUM International Group, LLC 2015-06-03 |
 MAXUM 86482995 4842795 Live/Registered |
EASTON DIAMOND SPORTS, LLC 2014-12-17 |
 MAXUM 81027052 1027052 Dead/Cancelled |
Sheffield Dairy Products Corporation 0000-00-00 |
 MAXUM 79109550 not registered Dead/Abandoned |
Siemens Aktiengesellschaft 2012-02-08 |
 MAXUM 78783015 not registered Dead/Abandoned |
Products Benefits Systems Corp. 2005-12-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.