LINK™ External Fixator C-20
GUDID M963C200
Soft Silicone Cover to use with LINK™ devices to protect from damage. This model is for use with parts 20mm in length, 2 Pin configuration. These parts are replacement/supplemental in case of damage to original cover provided with LINK™ device.
METRIC MEDICAL DEVICES, INC.
External orthopaedic fixation system, single-use| Primary Device ID | M963C200 |
| NIH Device Record Key | 60121a3c-a3bd-42ce-9808-0a5319f23cf8 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | LINK™ External Fixator |
| Version Model Number | C-20 |
| Catalog Number | C-20 |
| Company DUNS | 830255506 |
| Company Name | METRIC MEDICAL DEVICES, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | M963C200 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K240049
FDA Product Code
| KTT | Appliance, Fixation, Nail/Blade/Plate Combination, Multiple Component |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-06-17 |
| Device Publish Date | 2024-06-07 |
On-Brand Devices [LINK™ External Fixator]
| M963CH20100 | Soft Silicone Cover to use with LINK™ devices to protect from damage. This model is for use w |
| M963C200 | Soft Silicone Cover to use with LINK™ devices to protect from damage. This model is for use w |
| M9634H201020 | LINK™ External Fixator, 4 Pin, Square Configuration intended for assembly and use with appropr |
| M96322000200 | LINK™ External Fixator, 2 Pin, Straight Configuration intended for assembly and use with appro |
| M96320TT0 | 2.0 mm Diameter, Threaded Bone Pins as part of the LINK™ External Fixator, provided in a segre |
| M96320TG0 | 2.0 mm Diameter, Threaded and Grooved Bone Pins as part of the LINK™ External Fixator, provide |
| M96316TT0 | 1.6 mm Diameter, Threaded Bone Pins as part of the LINK™ External Fixator, provided in a segre |
Trademark Results [LINK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LINK 98858329 not registered Live/Pending |
Dzine Products, LLC 2024-11-18 |
 LINK 98848487 not registered Live/Pending |
Link Adventure Gear 2024-11-12 |
 LINK 98785976 not registered Live/Pending |
Leap Here LLC 2024-10-04 |
 LINK 98715583 not registered Live/Pending |
Link Management Systems Inc. 2024-08-24 |
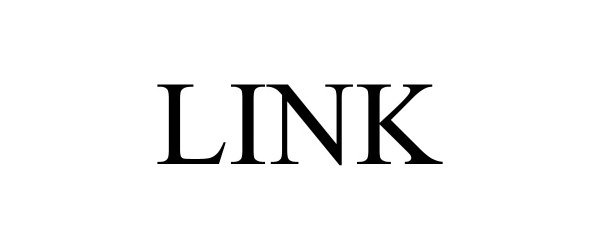 LINK 98604396 not registered Live/Pending |
FENIEX INDUSTRIES INC. 2024-06-17 |
 LINK 98507530 not registered Live/Pending |
Stripe, Inc. 2024-04-18 |
 LINK 98450996 not registered Live/Pending |
Smart Tracking Technologies, LLC 2024-03-15 |
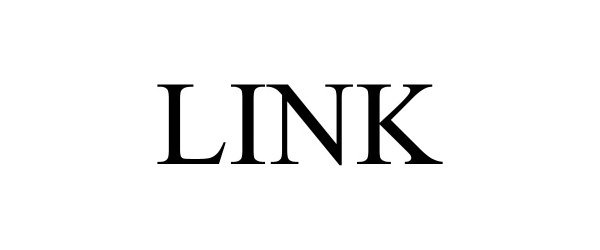 LINK 98157539 not registered Live/Pending |
Pradopark, S.A. 2023-08-30 |
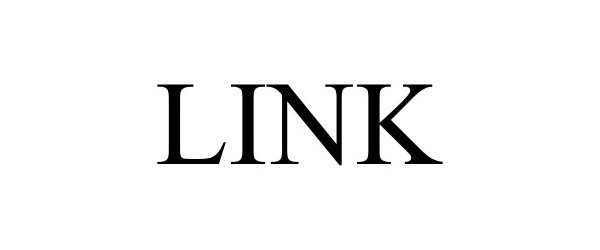 LINK 98090489 not registered Live/Pending |
REV Recreation Group, Inc. 2023-07-18 |
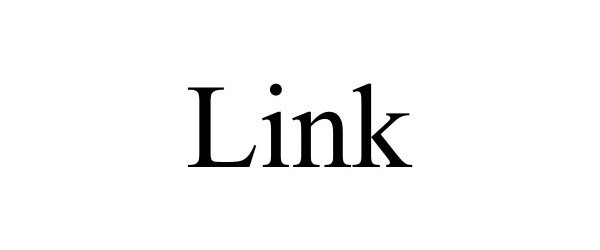 LINK 98031955 not registered Live/Pending |
Liminal Strategy Partners LLC 2023-06-07 |
 LINK 97873463 not registered Live/Pending |
Intrinsic Group Limited 2023-04-05 |
 LINK 97705709 not registered Live/Pending |
LINK S.R.L. 2022-12-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.