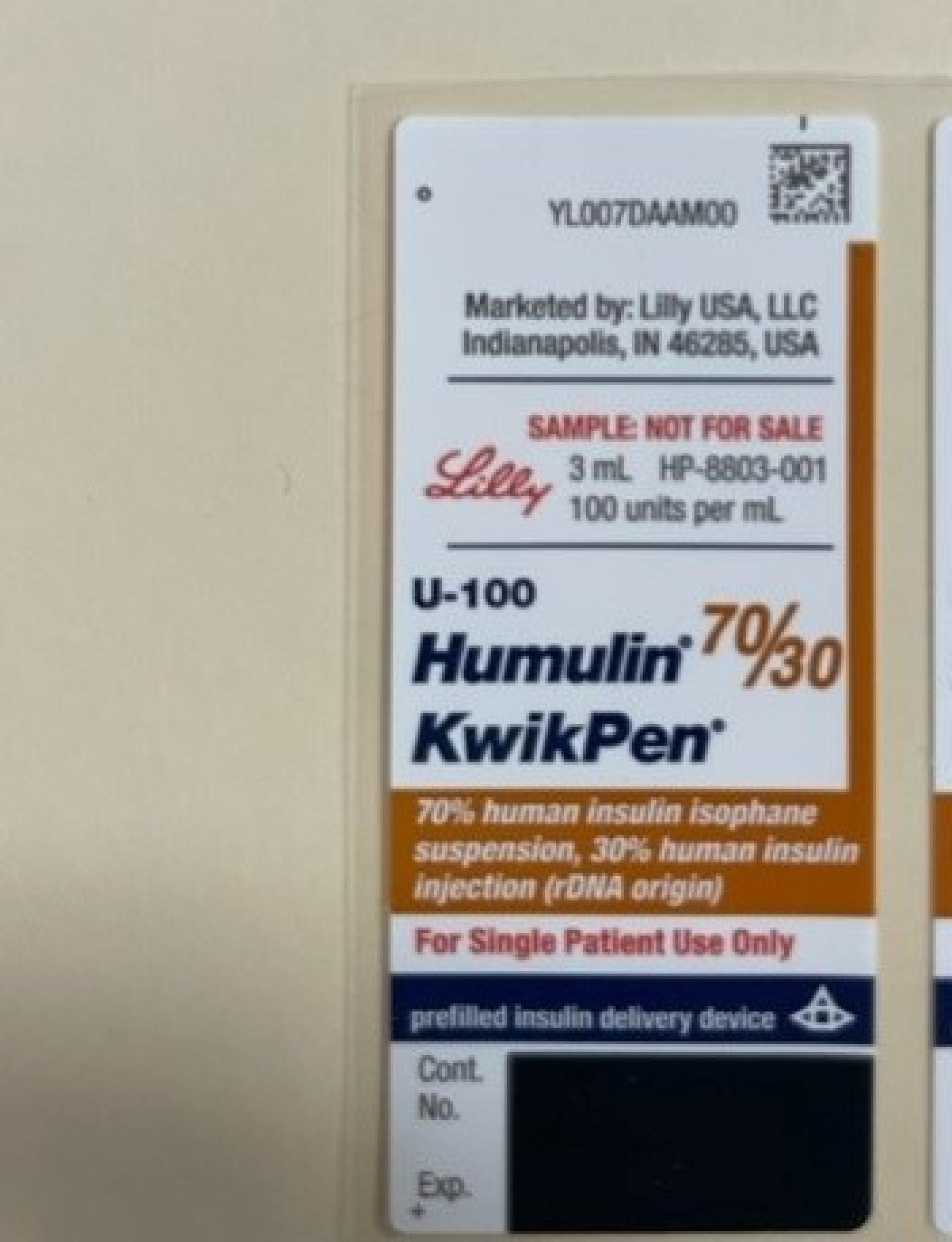
NDC 0002-8715
Humulin 70/30
Insulin Human
Humulin 70/30 is a Subcutaneous Injection, Suspension in the Human Otc Drug category. It is labeled and distributed by Eli Lilly And Company. The primary component is Insulin Human.
| Product ID | 0002-8715_404554e1-a052-45cc-b83c-e2828385df3a |
| NDC | 0002-8715 |
| Product Type | Human Otc Drug |
| Proprietary Name | Humulin 70/30 |
| Generic Name | Insulin Human |
| Dosage Form | Injection, Suspension |
| Route of Administration | SUBCUTANEOUS |
| Marketing Start Date | 1989-06-26 |
| Marketing Category | BLA / NDA |
| Application Number | BLA019717 |
| Labeler Name | Eli Lilly and Company |
| Substance Name | INSULIN HUMAN |
| Active Ingredient Strength | 100 [iU]/mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2022-12-31 |
Packaging
NDC 0002-8715-01
1 VIAL, MULTI-DOSE in 1 CARTON (0002-8715-01) > 10 mL in 1 VIAL, MULTI-DOSE
| Marketing Start Date | 1989-06-26 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 0002-8715-91 [00002871591]
Humulin 70/30 INJECTION, SUSPENSION
| Marketing Category | NDA |
| Application Number | NDA019717 |
| Product Type | HUMAN OTC DRUG |
| Billing Unit | ML |
| Marketing Start Date | 2010-06-10 |
| Marketing End Date | 2015-01-31 |
NDC 0002-8715-99 [00002871599]
Humulin 70/30 INJECTION, SUSPENSION
| Marketing Category | NDA |
| Application Number | NDA019717 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 1993-08-06 |
NDC 0002-8715-01 [00002871501]
Humulin 70/30 INJECTION, SUSPENSION
| Marketing Category | NDA |
| Application Number | NDA019717 |
| Product Type | HUMAN OTC DRUG |
| Billing Unit | ML |
| Marketing Start Date | 1989-06-26 |
NDC 0002-8715-17 [00002871517]
Humulin 70/30 INJECTION, SUSPENSION
| Marketing Category | NDA |
| Application Number | NDA019717 |
| Product Type | HUMAN OTC DRUG |
| Billing Unit | ML |
| Marketing Start Date | 2011-04-09 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| INSULIN HUMAN | 100 [iU]/mL |
OpenFDA Data
| SPL SET ID: | e245e0c5-b2d6-418b-baa4-1c3324292885 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
NDC Crossover Matching brand name "Humulin 70/30" or generic name "Insulin Human"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 50090-0444 | Humulin 70/30 | Humulin 70/30 |
| 47918-874 | Afrezza | Insulin Human |
| 0002-8215 | Humulin | Insulin human |
| 0002-8315 | Humulin | Insulin human |
| 0002-8501 | Humulin | Insulin human |
| 0002-8715 | Humulin | Insulin human |
| 0002-8803 | Humulin | Insulin human |
| 0002-8805 | Humulin | Insulin human |
| 0002-8824 | Humulin | Insulin human |
| 0338-0126 | MYXREDLIN | insulin human |
Trademark Results [Humulin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HUMULIN 75773392 2345798 Live/Registered |
Eli Lilly and Company 1999-08-11 |
 HUMULIN 75253619 not registered Dead/Abandoned |
Eli Lilly and Company 1997-03-07 |
 HUMULIN 73303147 1201754 Live/Registered |
Eli Lilly and Company 1981-03-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.