NDC 0023-0667
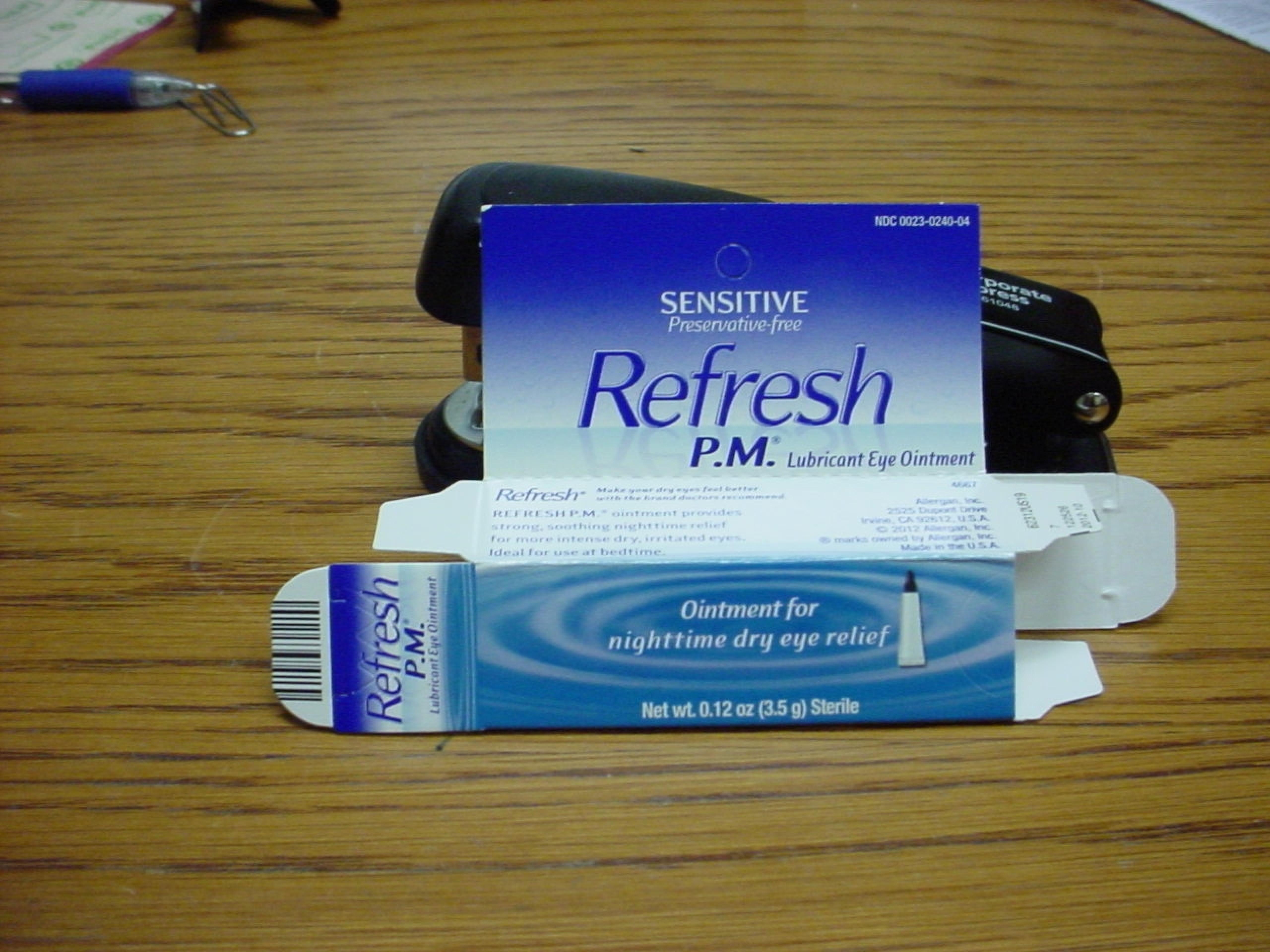
REFRESH P.M.
Mineral Oil, Petrolatum
REFRESH P.M. is a Ophthalmic Ointment in the Human Otc Drug category. It is labeled and distributed by Allergan, Inc.. The primary component is Mineral Oil; Petrolatum.
| Product ID | 0023-0667_599e3ac1-fd3e-4c0f-99d1-5161accb79cb |
| NDC | 0023-0667 |
| Product Type | Human Otc Drug |
| Proprietary Name | REFRESH P.M. |
| Generic Name | Mineral Oil, Petrolatum |
| Dosage Form | Ointment |
| Route of Administration | OPHTHALMIC |
| Marketing Start Date | 2016-08-26 |
| Marketing Category | OTC MONOGRAPH FINAL / |
| Application Number | part349 |
| Labeler Name | Allergan, Inc. |
| Substance Name | MINERAL OIL; PETROLATUM |
| Active Ingredient Strength | 425 mg/g; mg/g |
| NDC Exclude Flag | N |
| Listing Certified Through | 2023-12-31 |
Packaging
NDC 0023-0667-04
1 TUBE in 1 CARTON (0023-0667-04) > 3.5 g in 1 TUBE
| Marketing Start Date | 2016-08-26 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "REFRESH P.M." or generic name "Mineral Oil, Petrolatum"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 0023-0240 | REFRESH P.M. | mineral oil, petrolatum |
| 0023-0667 | REFRESH P.M. | mineral oil, petrolatum |
| 17312-401 | Com-Pare2 Nighttime Eye | mineral oil, petrolatum |
| 79903-026 | EQUATE EYE LUBRICANT | mineral oil, petrolatum |
| 0023-0312 | REFRESH LACRI-LUBE | mineral oil, petrolatum |
Trademark Results [REFRESH P.M.]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REFRESH P.M. 74289737 1765517 Live/Registered |
ALLERGAN, INC. 1992-06-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.