NDC 0065-0411
Betadine
Povidone-iodine
Betadine is a Ophthalmic Solution in the Human Prescription Drug category. It is labeled and distributed by Alcon Laboratories, Inc.. The primary component is Povidone-iodine.
| Product ID | 0065-0411_0ec586f4-3013-473c-8957-12b4a8101cc4 |
| NDC | 0065-0411 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Betadine |
| Generic Name | Povidone-iodine |
| Dosage Form | Solution |
| Route of Administration | OPHTHALMIC |
| Marketing Start Date | 2000-04-01 |
| Marketing Category | NDA / NDA |
| Application Number | NDA018634 |
| Labeler Name | Alcon Laboratories, Inc. |
| Substance Name | POVIDONE-IODINE |
| Active Ingredient Strength | 5 mg/mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2021-12-31 |
Packaging
NDC 0065-0411-30
1 BOTTLE in 1 CARTON (0065-0411-30) > 30 mL in 1 BOTTLE
| Marketing Start Date | 2000-04-01 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 0065-0411-30 [00065041130]
Betadine SOLUTION
| Marketing Category | NDA |
| Application Number | NDA018634 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | ML |
| Marketing Start Date | 2000-04-01 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| POVIDONE-IODINE | 5 mg/mL |
OpenFDA Data
| SPL SET ID: | b026de1b-9949-4557-ac49-c7b0038c24cd |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI | |
| UPC Code |
NDC Crossover Matching brand name "Betadine" or generic name "Povidone-iodine"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 0065-0411 | Betadine | povidone-iodine |
| 67618-148 | Betadine | Betadine |
| 67618-152 | Betadine | Betadine |
| 67618-160 | Betadine | Betadine |
| 42254-006 | Betadine | Betadine |
| 0363-0939 | Apothecary PVP Topical | Povidone-Iodine |
| 17271-205 | BD E-Z SCRUB | povidone-iodine |
| 17271-245 | BD E-Z SCRUB | povidone-iodine |
| 17271-503 | BD E-Z Scrub | Povidone-Iodine |
| 0363-0777 | First Aid Antiseptic | POVIDONE-IODINE |
| 0536-1271 | First Aid Antiseptic | POVIDONE-IODINE |
| 11673-003 | First Aid Antiseptic | POVIDONE-IODINE |
| 0924-8112 | First Aid Only Povidone-Iodine Antiseptic | Povidone-iodine |
| 0924-8113 | First Aid Only Povidone-Iodine Antiseptic | POVIDONE-IODINE |
| 0924-8110 | First Aid Only Povidone-Iodine Prep Pad | Povidone-Iodine |
| 0113-0110 | fresh and pure | Povidone-iodine |
| 0404-0121 | Henry Schein Povidone-Iodine Prep Pad | POVIDONE-IODINE |
| 0404-0119 | Henry Schein Povidone-Iodine Swabsticks | POVIDONE-IODINE |
| 0404-0120 | Henry Schein Povidone-Iodine Swabsticks | POVIDONE-IODINE |
| 0904-1102 | Major Povidone Iodine | POVIDONE-IODINE |
| 0498-0118 | North PVP Iodine Swabs | Povidone-iodine |
| 0869-0050 | Povidone Iodine Solution First Aid Antiseptic | POVIDONE-IODINE |
| 0924-8111 | Povidone-Iodine Prep | POVIDONE-IODINE |
| 0498-0128 | PVP Iodine Swabs | Povidone-iodine |
| 0132-8743 | Summers Eve | POVIDONE-IODINE |
Trademark Results [Betadine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BETADINE 90851747 not registered Live/Pending |
Avrio Health L.P. 2021-07-27 |
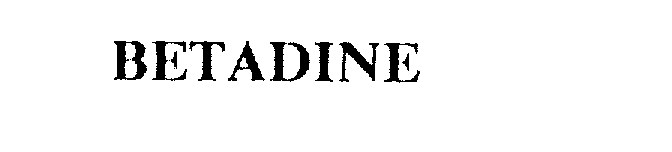 BETADINE 75438619 2289922 Live/Registered |
AVRIO HEALTH L.P. 1998-02-23 |
 BETADINE 75429382 not registered Dead/Abandoned |
PURDUE FREDERICK COMPANY, THE 1998-02-05 |
 BETADINE 75429381 2649701 Dead/Cancelled |
PURDUE FREDERICK COMPANY, THE 1998-02-05 |
 BETADINE 75429376 2225977 Live/Registered |
AVRIO HEALTH L.P. 1998-02-05 |
 BETADINE 75000809 1984349 Dead/Cancelled |
PURDUE FREDERICK COMPANY, THE 1995-10-02 |
 BETADINE 74711684 1984228 Dead/Cancelled |
PURDUE FREDERICK COMPANY, THE 1995-08-07 |
 BETADINE 74596982 1982032 Dead/Cancelled |
Purdue Frederick Company, The 1994-11-09 |
 BETADINE 73839535 1601849 Live/Registered |
PURDUE FREDERICK COMPANY 1989-11-15 |
 BETADINE 73779372 1559504 Live/Registered |
PURDUE FREDERICK COMPANY, THE 1989-02-06 |
 BETADINE 73107412 1069261 Dead/Expired |
PURDUE FREDERICK COMPANY, THE 1976-11-22 |
 BETADINE 73072465 1059771 Dead/Expired |
PURDUE FREDERICK COMPANY, THE 1975-12-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.