NDC 0069-3070
Zithromax
Azithromycin Dihydrate
Zithromax is a Oral Tablet, Film Coated in the Human Prescription Drug category. It is labeled and distributed by Pfizer Laboratories Div Pfizer Inc. The primary component is Azithromycin Dihydrate.
| Product ID | 0069-3070_0a7a028c-ef61-4dcc-8ab6-f20cf8270a86 |
| NDC | 0069-3070 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Zithromax |
| Generic Name | Azithromycin Dihydrate |
| Dosage Form | Tablet, Film Coated |
| Route of Administration | ORAL |
| Marketing Start Date | 2002-05-24 |
| Marketing Category | NDA / NDA |
| Application Number | NDA050784 |
| Labeler Name | Pfizer Laboratories Div Pfizer Inc |
| Substance Name | AZITHROMYCIN DIHYDRATE |
| Active Ingredient Strength | 500 mg/1 |
| Pharm Classes | Macrolide Antimicrobial [EPC],Macrolides [CS] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2021-12-31 |
Packaging
NDC 0069-3070-30
30 TABLET, FILM COATED in 1 BOTTLE (0069-3070-30)
| Marketing Start Date | 2002-05-24 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
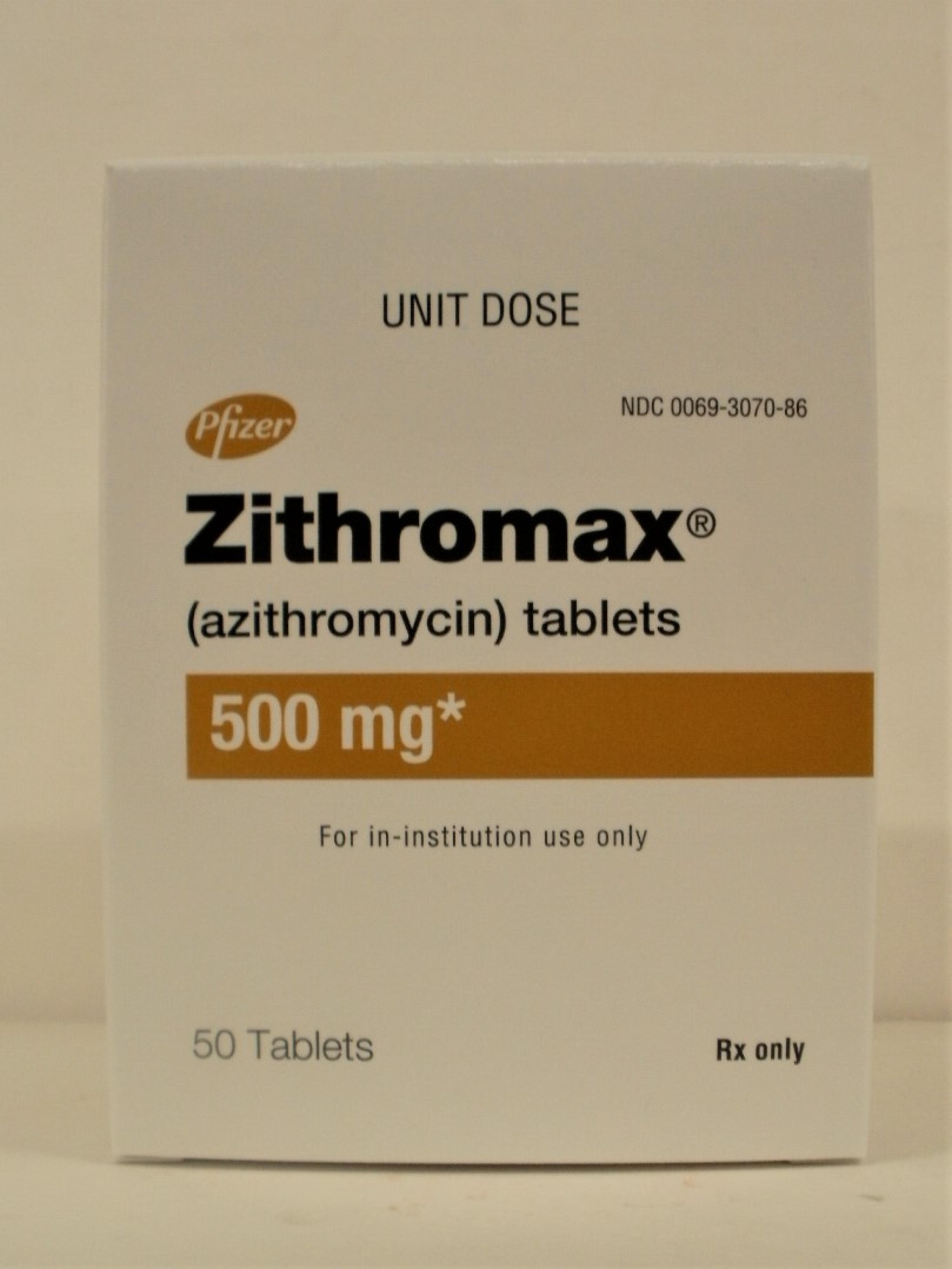
NDC 0069-3070-86 [00069307086]
Zithromax TABLET, FILM COATED
| Marketing Category | NDA |
| Application Number | NDA050784 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2002-05-24 |
NDC 0069-3070-30 [00069307030]
Zithromax TABLET, FILM COATED
| Marketing Category | NDA |
| Application Number | NDA050784 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2002-05-24 |
NDC 0069-3070-75 [00069307075]
Zithromax TABLET, FILM COATED
| Marketing Category | NDA |
| Application Number | NDA050784 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2002-05-24 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| AZITHROMYCIN DIHYDRATE | 500 mg/1 |
OpenFDA Data
| SPL SET ID: | db52b91e-79f7-4cc1-9564-f2eee8e31c45 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI | |
| UPC Code |
Pharmacological Class
- Macrolide Antimicrobial [EPC]
- Macrolides [CS]
NDC Crossover Matching brand name "Zithromax" or generic name "Azithromycin Dihydrate"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 0069-0400 | Zithromax | azithromycin dihydrate |
| 0069-3051 | ZITHROMAX | AZITHROMYCIN DIHYDRATE |
| 0069-3060 | Zithromax | azithromycin dihydrate |
| 0069-3070 | Zithromax | azithromycin dihydrate |
| 0069-3080 | ZITHROMAX | AZITHROMYCIN DIHYDRATE |
| 0069-3110 | Zithromax | azithromycin dihydrate |
| 70518-0249 | Zithromax | Zithromax |
| 70518-0336 | Zithromax | Zithromax |
| 70518-0505 | Zithromax | Zithromax |
| 0069-3120 | Zithromax | Zithromax |
| 0069-3130 | Zithromax | Zithromax |
| 0069-3140 | Zithromax | Zithromax |
| 0069-3150 | Zithromax | Zithromax |
| 50090-0603 | Zithromax | Zithromax |
| 55154-2713 | Zithromax | Zithromax |
| 61919-555 | ZITHROMAX | ZITHROMAX |
| 0069-4061 | Zithromax | Zithromax |
| 0069-9500 | Zithromax | Zithromax |
Trademark Results [Zithromax]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZITHROMAX 74034510 1702392 Live/Registered |
PFIZER INC. 1990-03-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.