NDC 0093-5454
Mimvey Lo
Estradiol And Norethindrone Acetate
Mimvey Lo is a Oral Tablet in the Human Prescription Drug category. It is labeled and distributed by Teva Pharmaceuticals Usa,. The primary component is Estradiol; Nore.
| Product ID | 0093-5454_b3dd25ea-5ccb-45f2-a567-f00267177df7 |
| NDC | 0093-5454 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Mimvey Lo |
| Generic Name | Estradiol And Norethindrone Acetate |
| Dosage Form | Tablet |
| Route of Administration | ORAL |
| Marketing Start Date | 2014-06-02 |
| Marketing Category | ANDA / ANDA |
| Application Number | ANDA200747 |
| Labeler Name | Teva Pharmaceuticals USA, |
| Substance Name | ESTRADIOL; NORE |
| Active Ingredient Strength | 1 mg/1; mg/1 |
| Pharm Classes | Estradiol Conge |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 0093-5454-28
1 BLISTER PACK in 1 CARTON (0093-5454-28) > 28 TABLET in 1 BLISTER PACK
| Marketing Start Date | 2015-04-08 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 0093-5454-62 [00093545462]
Mimvey Lo TABLET
| Marketing Category | ANDA |
| Application Number | ANDA200747 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2014-06-02 |
| Marketing End Date | 2018-05-31 |
NDC 0093-5454-28 [00093545428]
Mimvey Lo TABLET
| Marketing Category | ANDA |
| Application Number | ANDA200747 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2015-04-08 |
| Marketing End Date | 2015-04-08 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| ESTRADIOL | .5 mg/1 |
OpenFDA Data
| SPL SET ID: | b1c5c06b-9ce2-4bf7-9693-e37608055a14 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
Pharmacological Class
- Estradiol Congeners [CS]
- Estrogen [EPC]
- Estrogen Receptor Agonists [MoA]
- Progesterone Congeners [CS]
- Progestin [EPC]
NDC Crossover Matching brand name "Mimvey Lo" or generic name "Estradiol And Norethindrone Acetate"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 0093-5454 | Mimvey Lo | Estradiol and Norethindrone Acetate |
| 60846-201 | Activella | estradiol and norethindrone acetate |
| 60846-202 | Activella | estradiol and norethindrone acetate |
| 68180-829 | AMABELZ | estradiol and norethindrone acetate |
| 68180-830 | AMABELZ | estradiol and norethindrone acetate |
| 51991-474 | Estradiol / Norethindrone Acetate | Estradiol and Norethindrone Acetate |
| 51991-623 | Estradiol / Norethindrone Acetate | Estradiol and Norethindrone Acetate |
| 0378-7294 | ESTRADIOL AND NORETHINDRONE ACETATE | estradiol and norethindrone acetate |
| 0378-7295 | ESTRADIOL AND NORETHINDRONE ACETATE | estradiol and norethindrone acetate |
| 50742-657 | Estradiol and Norethindrone Acetate | Estradiol and Norethindrone Acetate |
| 50742-658 | Estradiol and Norethindrone Acetate | Estradiol and Norethindrone Acetate |
| 0093-5455 | Mimvey | Estradiol and Norethindrone Acetate |
Trademark Results [Mimvey Lo]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
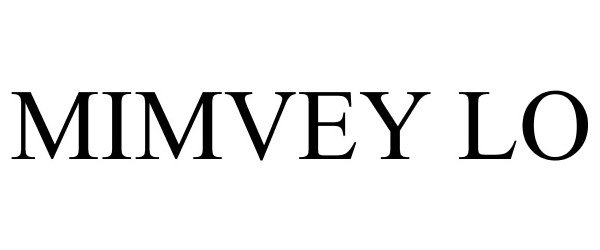 MIMVEY LO 85373764 4597505 Live/Registered |
Teva Pharmaceuticals USA, Inc. 2011-07-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.