NDC 0480-4490
Tasimelteon
Tasimelteon
Tasimelteon is a Oral Capsule, Gelatin Coated in the Human Prescription Drug category. It is labeled and distributed by Teva Pharmaceuticals, Inc.. The primary component is Tasimelteon.
| Product ID | 0480-4490_64e7208e-cb62-44ec-b4bf-6b39f2500259 |
| NDC | 0480-4490 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Tasimelteon |
| Generic Name | Tasimelteon |
| Dosage Form | Capsule, Gelatin Coated |
| Route of Administration | ORAL |
| Marketing Start Date | 2022-12-29 |
| Marketing Category | ANDA / |
| Application Number | ANDA211601 |
| Labeler Name | Teva Pharmaceuticals, Inc. |
| Substance Name | TASIMELTEON |
| Active Ingredient Strength | 20 mg/1 |
| Pharm Classes | Melatonin Receptor Agonist [EPC], Melatonin Receptor Agonists [MoA] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2024-12-31 |
Packaging
NDC 0480-4490-56
30 CAPSULE, GELATIN COATED in 1 BOTTLE (0480-4490-56)
| Marketing Start Date | 2022-12-29 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "Tasimelteon" or generic name "Tasimelteon"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 0480-4490 | Tasimelteon | Tasimelteon |
| 43068-220 | Hetlioz | tasimelteon |
| 43068-304 | Hetlioz | tasimelteon |
Trademark Results [Tasimelteon]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
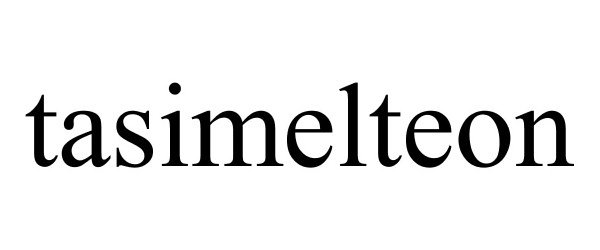 TASIMELTEON 77160897 not registered Dead/Abandoned |
Vanda Pharmaceuticals Inc. 2007-04-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.