NDC 0944-2512
HYQVIA
Immune Globulin 10 Percent (human) With Recombinant Human Hyaluronidase
HYQVIA is a Kit in the Plasma Derivative category. It is labeled and distributed by Baxalta Us Inc.. The primary component is .
| Product ID | 0944-2512_1283c294-04ea-4a13-80b4-37e3a023ceca |
| NDC | 0944-2512 |
| Product Type | Plasma Derivative |
| Proprietary Name | HYQVIA |
| Generic Name | Immune Globulin 10 Percent (human) With Recombinant Human Hyaluronidase |
| Dosage Form | Kit |
| Marketing Start Date | 2014-09-12 |
| Marketing Category | BLA / BLA |
| Application Number | BLA125402 |
| Labeler Name | Baxalta US Inc. |
| Active Ingredient Strength | 0 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2022-12-31 |
Packaging
NDC 0944-2512-02
1 KIT in 1 CARTON (0944-2512-02) * 100 mL in 1 VIAL, GLASS (0944-2717-10) * 5 mL in 1 VIAL, GLASS (0944-2722-03)
| Marketing Start Date | 2014-09-12 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 0944-2512-02 [00944251202]
HYQVIA KIT
| Marketing Category | BLA |
| Application Number | BLA125402 |
| Product Type | PLASMA DERIVATIVE |
| Billing Unit | ML |
| Marketing Start Date | 2014-09-12 |
Drug Details
NDC Crossover Matching brand name "HYQVIA" or generic name "Immune Globulin 10 Percent (human) With Recombinant Human Hyaluronidase"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 0944-2510 | HYQVIA | Immune Globulin 10 percent (Human) with Recombinant Human Hyaluronidase |
| 0944-2511 | HYQVIA | Immune Globulin 10 percent (Human) with Recombinant Human Hyaluronidase |
| 0944-2512 | HYQVIA | Immune Globulin 10 percent (Human) with Recombinant Human Hyaluronidase |
| 0944-2513 | HYQVIA | Immune Globulin 10 percent (Human) with Recombinant Human Hyaluronidase |
| 0944-2514 | HYQVIA | Immune Globulin 10 percent (Human) with Recombinant Human Hyaluronidase |
Trademark Results [HYQVIA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
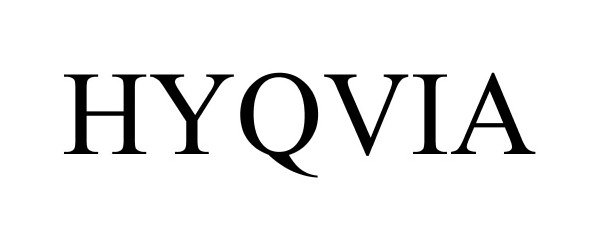 HYQVIA 86348519 4774188 Live/Registered |
BAXALTA INCORPORATED 2014-07-25 |
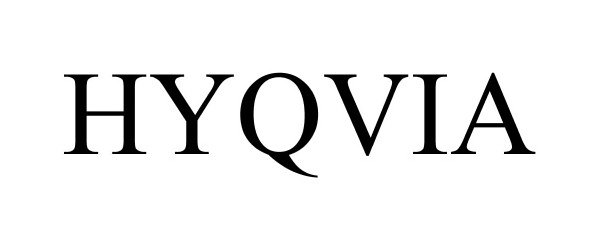 HYQVIA 85239796 4669391 Live/Registered |
BAXALTA INCORPORATED 2011-02-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.