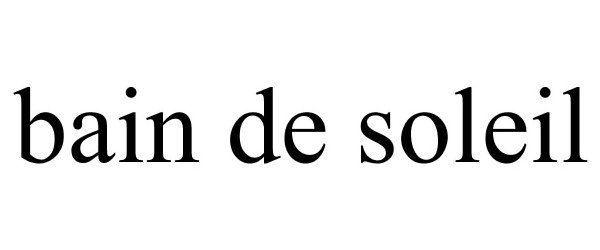| NDC | Brand Name | Generic Name |
|---|
| 11523-7189 | Bain de Soleil | Octinoxate |
| 10096-0038 | Beyond Color | OCTINOXATE |
| 10096-0321 | Beyond Color | OCTINOXATE |
| 10096-0328 | Beyond Color | OCTINOXATE |
| 10096-0329 | Beyond Color | OCTINOXATE |
| 0869-0503 | Complete Beauty | Octinoxate |
| 11559-732 | DOUBLE MATTE | OCTINOXATE |
| 13537-001 | ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 | Octinoxate |
| 13537-002 | ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 | Octinoxate |
| 13537-003 | ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 | Octinoxate |
| 13537-004 | ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 | Octinoxate |
| 13537-005 | ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 | Octinoxate |
| 13537-006 | ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 | Octinoxate |
| 13537-007 | ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 | Octinoxate |
| 13537-008 | ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 | Octinoxate |
| 13537-009 | ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 | Octinoxate |
| 13537-016 | ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 | Octinoxate |
| 13537-017 | ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 | Octinoxate |
| 13537-018 | ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 | Octinoxate |
| 13537-024 | ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 | Octinoxate |
| 13537-029 | ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 | Octinoxate |
| 13537-030 | ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 | Octinoxate |
| 13537-180 | ESIKA PRO PERFECT MATCH VOLUMEN | Octinoxate |
| 13537-181 | ESIKA PRO PERFECT MATCH VOLUMEN | Octinoxate |
| 13537-182 | ESIKA PRO PERFECT MATCH VOLUMEN | Octinoxate |
| 13537-183 | ESIKA PRO PERFECT MATCH VOLUMEN | Octinoxate |
| 13537-184 | ESIKA PRO PERFECT MATCH VOLUMEN | Octinoxate |
| 13537-185 | ESIKA PRO PERFECT MATCH VOLUMEN | Octinoxate |
| 13537-186 | ESIKA PRO PERFECT MATCH VOLUMEN | Octinoxate |
| 10096-0215 | Healthy Makeup | OCTINOXATE |
| 10096-0216 | Healthy Makeup | OCTINOXATE |
| 13537-200 | LBEL FILLING EFFECT FOUNDATION SPF 10 | Octinoxate |
| 13537-201 | LBEL FILLING EFFECT FOUNDATION SPF 10 | Octinoxate |
| 13537-202 | LBEL FILLING EFFECT FOUNDATION SPF 10 | Octinoxate |
| 13537-203 | LBEL FILLING EFFECT FOUNDATION SPF 10 | Octinoxate |
| 11559-725 | LIP CONDITIONER SPF 15 | OCTINOXATE |
| 10096-0261 | Satin Satisfaction | OCTINOXATE |
| 10096-0325 | ultra color absolute | Octinoxate |
| 10096-0191 | Ultra Color Rich | OCTINOXATE |
| 10096-0229 | Ultra Color Rich | OCTINOXATE |
| 0363-4004 | Walgreens Dark Tanning Oil Sunscreen | OCTINOXATE |