NDC 11673-371
Aspirin Enteric Coated
Aspirin
Aspirin Enteric Coated is a Oral Tablet, Coated in the Human Otc Drug category. It is labeled and distributed by Target Corporation. The primary component is Aspirin.
| Product ID | 11673-371_ac915a2d-ace2-429c-8335-604bf111d06a |
| NDC | 11673-371 |
| Product Type | Human Otc Drug |
| Proprietary Name | Aspirin Enteric Coated |
| Generic Name | Aspirin |
| Dosage Form | Tablet, Coated |
| Route of Administration | ORAL |
| Marketing Start Date | 2011-04-05 |
| Marketing End Date | 2020-12-31 |
| Marketing Category | OTC MONOGRAPH FINAL / OTC MONOGRAPH FINAL |
| Application Number | part343 |
| Labeler Name | TARGET Corporation |
| Substance Name | ASPIRIN |
| Active Ingredient Strength | 325 mg/1 |
| NDC Exclude Flag | N |
Packaging
NDC 11673-371-30
1 BOTTLE, PLASTIC in 1 BOX (11673-371-30) > 300 TABLET, COATED in 1 BOTTLE, PLASTIC
| Marketing Start Date | 2011-04-05 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 11673-371-30 [11673037130]
Aspirin Enteric Coated TABLET, COATED
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part343 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2011-04-05 |
| Marketing End Date | 2020-12-31 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| ASPIRIN | 325 mg/1 |
OpenFDA Data
| SPL SET ID: | 29990f6c-2db0-4eea-a106-70509580bb4e |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI | |
| Pharm Class PE | |
| PHarm Class EPC | |
| UPC Code | |
| NUI Code |
NDC Crossover Matching brand name "Aspirin Enteric Coated" or generic name "Aspirin"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 68016-089 | Aspirin Enteric Coated | Aspirin Enteric Coated |
| 69168-331 | Aspirin Enteric Coated | Aspirin Enteric Coated |
| 69168-318 | ASPIRIN Enteric Coated | ASPIRIN Enteric Coated |
| 11673-371 | Aspirin Enteric Coated | Aspirin Enteric Coated |
| 55315-103 | Aspirin Enteric Coated | Aspirin Enteric Coated |
| 55312-440 | Aspirin Enteric Coated | Aspirin Enteric Coated |
| 56062-440 | Aspirin Enteric Coated | Aspirin Enteric Coated |
| 59779-600 | Aspirin Enteric Coated | Aspirin Enteric Coated |
| 63868-363 | Aspirin Enteric Coated | Aspirin Enteric Coated |
| 69168-400 | Aspirin Enteric Coated | Aspirin Enteric Coated |
| 69168-372 | Aspirin Enteric Coated | Aspirin Enteric Coated |
| 0363-0157 | Aspirin | Aspirin |
| 0363-0218 | Aspirin | Aspirin |
| 0363-0227 | Aspirin | Aspirin |
| 0113-0773 | Basic Care Aspirin | Aspirin |
| 0113-7467 | basic care aspirin | Aspirin |
| 0280-2100 | Bayer | Aspirin |
| 0280-2110 | Bayer Aspirin Regimen | Aspirin |
| 0280-2080 | Bayer Aspirin Regimen Chewable | Aspirin |
| 0280-2090 | Bayer Chewable - Aspirin Regimen | Aspirin |
| 0280-2000 | Bayer Genuine Aspirin | Aspirin |
| 0280-2040 | Bayer Plus | Aspirin |
| 0280-2200 | Bayer Womens | Aspirin |
| 0067-6424 | BUFFERIN | ASPIRIN |
| 0113-0259 | good sense aspirin | Aspirin |
| 0113-0274 | Good Sense aspirin | Aspirin |
| 0113-0416 | good sense aspirin | Aspirin |
| 0113-0467 | good sense aspirin | Aspirin |
| 0113-1919 | Good Sense Aspirin | aspirin |
| 0363-0183 | Regular Strength Tri-Buffered Aspirin | Aspirin |
Trademark Results [Aspirin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
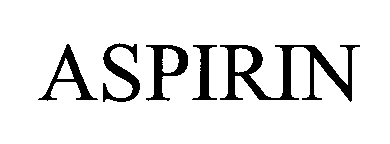 ASPIRIN 76481358 2788617 Dead/Cancelled |
Simon Carter Accessories, Ltd. 2003-01-10 |
 ASPIRIN 75209895 not registered Dead/Abandoned |
Bayer Aktiengesellschaft 1996-12-09 |
 ASPIRIN 73234351 1171777 Dead/Cancelled |
McIntyre; William A. 1979-10-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.