NDC 13672-051
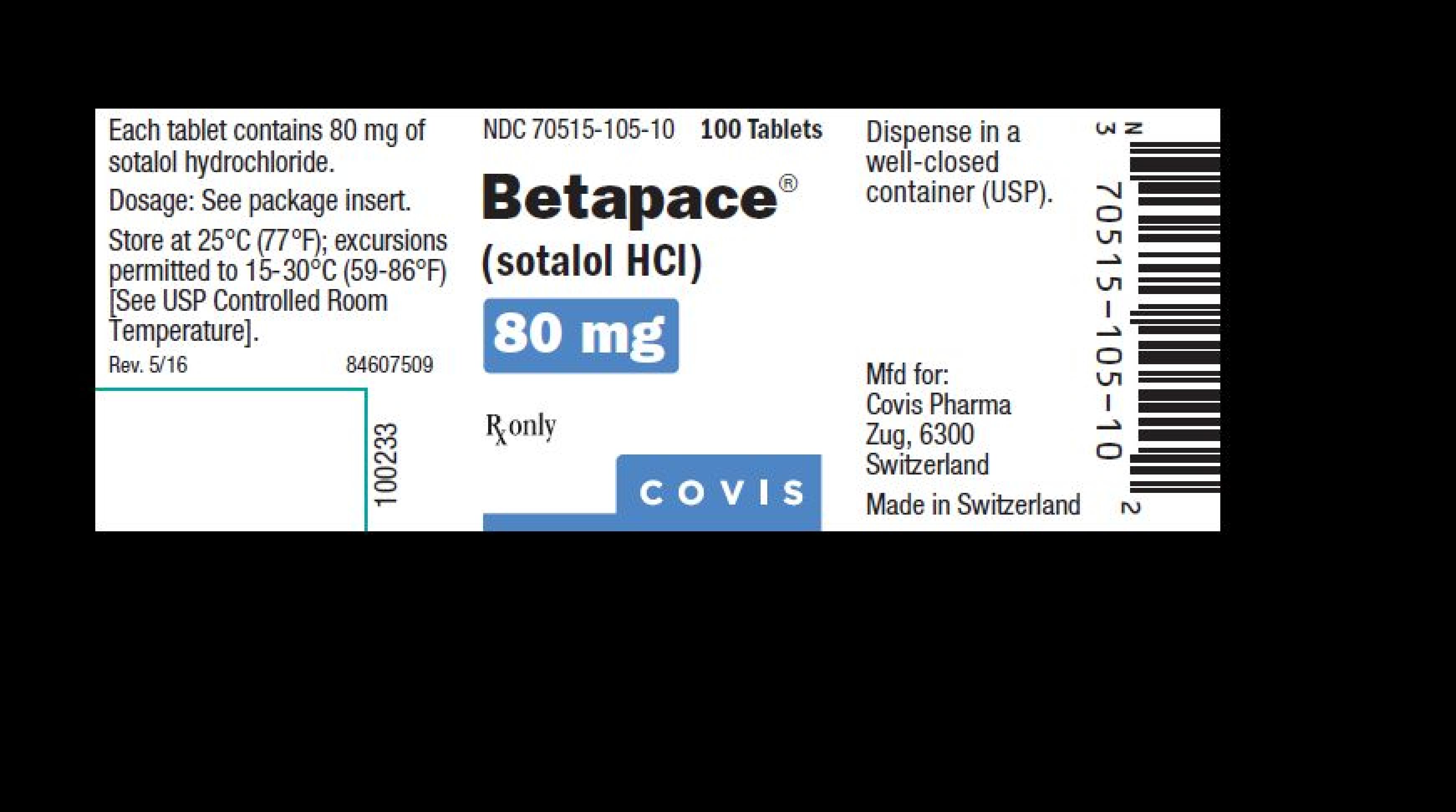
Betapace
Sotalol Hcl
Betapace is a Oral Tablet in the Human Prescription Drug category. It is labeled and distributed by Skyepharma Production Sas. The primary component is Sotalol Hydrochloride.
| Product ID | 13672-051_754a6348-953f-4e8e-e053-2991aa0adde2 |
| NDC | 13672-051 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Betapace |
| Generic Name | Sotalol Hcl |
| Dosage Form | Tablet |
| Route of Administration | ORAL |
| Marketing Start Date | 1992-10-30 |
| Marketing Category | NDA / NDA |
| Application Number | NDA019865 |
| Labeler Name | SkyePharma Production SAS |
| Substance Name | SOTALOL HYDROCHLORIDE |
| Active Ingredient Strength | 80 mg/80mg |
| Pharm Classes | Antiarrhythmic [EPC],Cardiac Rhythm Alteration [PE],Adrenergic beta-Antagonists [MoA] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 13672-051-00
100 mg in 1 BOTTLE (13672-051-00)
| Marketing Start Date | 1992-10-30 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 13672-051-00 [13672005100]
Betapace TABLET
| Marketing Category | NDA |
| Application Number | NDA019865 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Marketing Start Date | 1992-10-30 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| SOTALOL HYDROCHLORIDE | 80 mg/80mg |
Pharmacological Class
- Antiarrhythmic [EPC]
- Cardiac Rhythm Alteration [PE]
- Adrenergic beta-Antagonists [MoA]
NDC Crossover Matching brand name "Betapace" or generic name "Sotalol Hcl"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 13672-051 | Betapace | sotalol HCl |
| 13672-052 | Betapace | sotalol HCl |
| 13672-053 | Betapace | sotalol HCl |
| 70515-105 | BETAPACE | sotalol hydrochloride |
| 70515-106 | BETAPACE | sotalol hydrochloride |
| 70515-109 | BETAPACE | sotalol hydrochloride |
| 13672-054 | Betapace AF | sotalol HCl |
| 13672-055 | Betapace AF | sotalol HCl |
| 13672-056 | Betapace AF | sotalol HCl |
Trademark Results [Betapace]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BETAPACE 73701175 1504551 Live/Registered |
BRISTOL-MYERS COMPANY 1987-12-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.