NDC 17478-404
FUL-GLO
Fluorescein Sodium
FUL-GLO is a Ophthalmic Strip in the Human Prescription Drug category. It is labeled and distributed by Akorn. The primary component is Fluorescein Sodium.
| Product ID | 17478-404_2dc13f8d-ae12-44e5-90f3-f8fbbff0f4e3 |
| NDC | 17478-404 |
| Product Type | Human Prescription Drug |
| Proprietary Name | FUL-GLO |
| Generic Name | Fluorescein Sodium |
| Dosage Form | Strip |
| Route of Administration | OPHTHALMIC |
| Marketing Start Date | 2004-06-01 |
| Marketing Category | UNAPPROVED DRUG OTHER / UNAPPROVED DRUG OTHER |
| Labeler Name | Akorn |
| Substance Name | FLUORESCEIN SODIUM |
| Active Ingredient Strength | 1 mg/1 |
| Pharm Classes | Diagnostic Dye [EPC], Dyes [MoA] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2023-12-31 |
Packaging
NDC 17478-404-01
100 APPLICATOR in 1 CARTON (17478-404-01) > 1 STRIP in 1 APPLICATOR
| Marketing Start Date | 2004-06-01 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 17478-404-01 [17478040401]
FUL-GLO STRIP
| Marketing Category | Unapproved drug other |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2004-06-01 |
| Inactivation Date | 2019-11-13 |
| Reactivation Date | 2020-01-14 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| D&C YELLOW NO. 8 | 1 mg/1 |
OpenFDA Data
| SPL SET ID: | b297d7a1-2fc4-4e4f-9821-2a1985b61ae0 |
| Manufacturer |
Pharmacological Class
- Diagnostic Dye [EPC]
- Dyes [MoA]
NDC Crossover Matching brand name "FUL-GLO" or generic name "Fluorescein Sodium"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 17478-403 | FUL-GLO | Fluorescein Sodium |
| 17478-404 | FUL-GLO | Fluorescein Sodium |
| 17478-250 | AK-FLUOR | fluorescein sodium |
| 17478-253 | AK-FLUOR | fluorescein sodium |
| 50090-4535 | AK-FLUOR | fluorescein sodium |
| 17238-900 | BioGlo | Fluorescein Sodium |
| 51801-008 | Dry Eye Test | Fluorescein Sodium |
| 0065-0092 | FLUORESCITE | fluorescein sodium |
| 51801-003 | GloStrips | Fluorescein Sodium |
| 51801-009 | GloStrips | Fluorescein Sodium |
Trademark Results [FUL-GLO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 FUL-GLO 73436222 1304640 Dead/Cancelled |
Barnes-Hind/Hydrocurve Inc. 1983-07-25 |
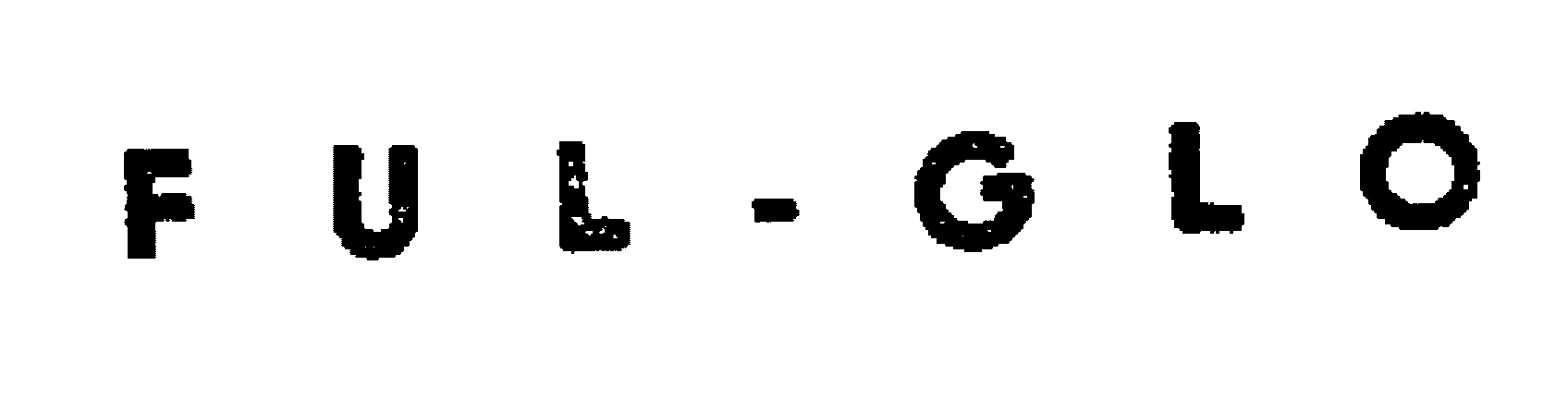 FUL-GLO 72097612 0709542 Dead/Abandoned |
BARNES-HIND OPHTHALMIC PRODUCTS, INC. 1960-05-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.