NDC 21695-941
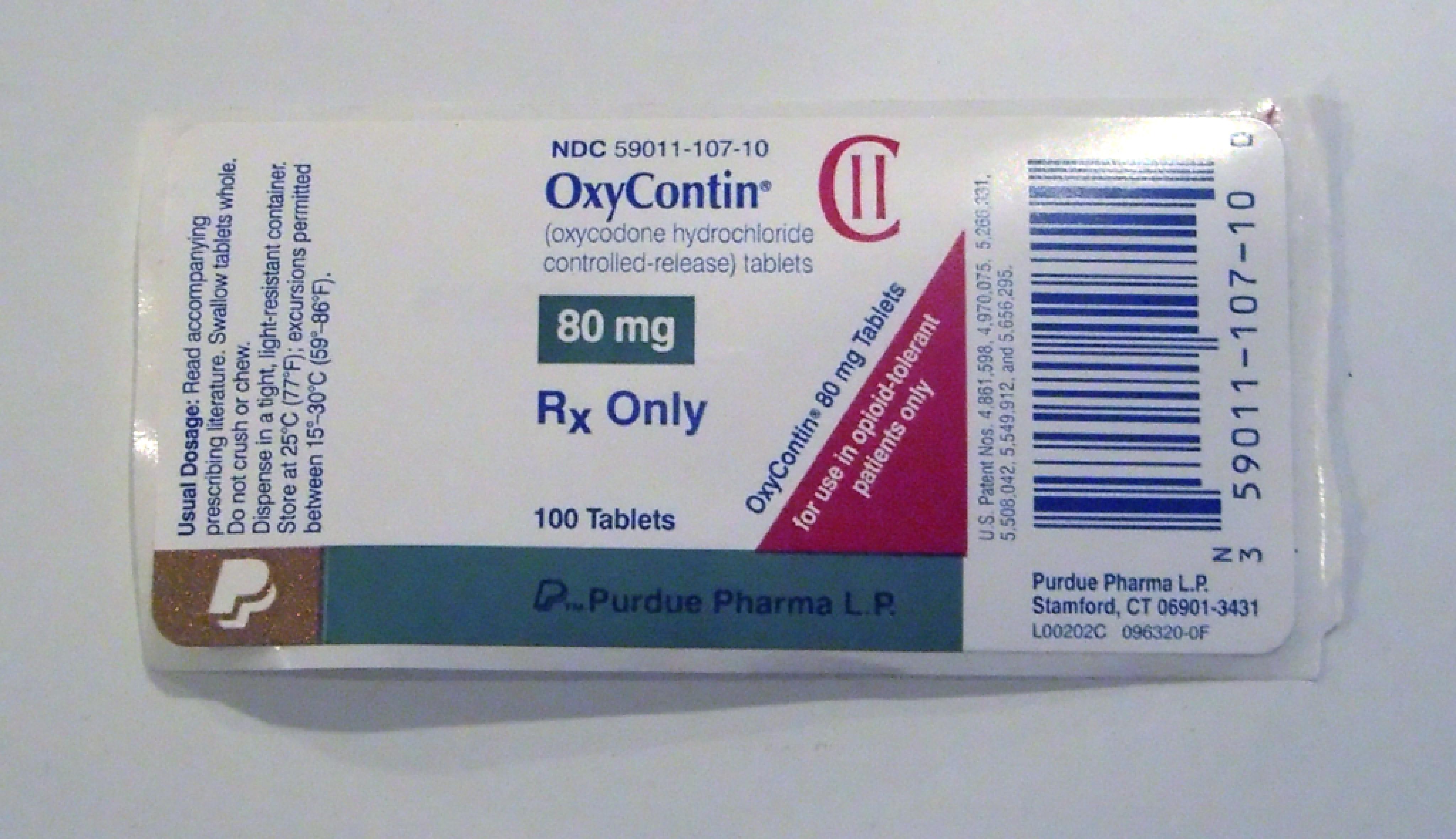
OxyContin
Oxycodone Hydrochloride
OxyContin is a Oral Tablet, Film Coated, Extended Release in the Human Prescription Drug category. It is labeled and distributed by Rebel Distributors Corp. The primary component is Oxycodone Hydrochloride.
| Product ID | 21695-941_8c9cb953-0995-4f47-83ee-c8c9dfd1b533 |
| NDC | 21695-941 |
| Product Type | Human Prescription Drug |
| Proprietary Name | OxyContin |
| Generic Name | Oxycodone Hydrochloride |
| Dosage Form | Tablet, Film Coated, Extended Release |
| Route of Administration | ORAL |
| Marketing Start Date | 2010-08-08 |
| Marketing Category | NDA / NDA |
| Application Number | NDA022272 |
| Labeler Name | Rebel Distributors Corp |
| Substance Name | OXYCODONE HYDROCHLORIDE |
| Active Ingredient Strength | 80 mg/1 |
| Pharm Classes | Full Opioid Agonists [MoA],Opioid Agonist [EPC] |
| DEA Schedule | CII |
| NDC Exclude Flag | E |
| Listing Certified Through | 2017-12-31 |
Packaging
NDC 21695-941-60
60 TABLET, FILM COATED, EXTENDED RELEASE in 1 BOTTLE (21695-941-60)
| Marketing Start Date | 2010-08-08 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 21695-941-60 [21695094160]
OxyContin TABLET, FILM COATED, EXTENDED RELEASE
| Marketing Category | NDA |
| Application Number | NDA022272 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2010-08-08 |
| Inactivation Date | 2019-09-24 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| OXYCODONE HYDROCHLORIDE | 80 mg/1 |
OpenFDA Data
| SPL SET ID: | 8c9cb953-0995-4f47-83ee-c8c9dfd1b533 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
Pharmacological Class
- Full Opioid Agonists [MoA]
- Opioid Agonist [EPC]
NDC Crossover Matching brand name "OxyContin" or generic name "Oxycodone Hydrochloride"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 21695-951 | OxyContin | OxyContin |
| 21695-941 | OxyContin | OxyContin |
| 42254-159 | OxyContin | OxyContin |
| 59011-410 | OxyContin | OxyContin |
| 59011-415 | OxyContin | OxyContin |
| 59011-430 | OxyContin | OxyContin |
| 59011-440 | OxyContin | OxyContin |
| 59011-480 | OxyContin | OxyContin |
| 59011-460 | OxyContin | OxyContin |
| 59011-420 | OxyContin | OxyContin |
| 63629-3774 | OxyContin | OxyContin |
| 0054-0390 | oxycodone hydrochloride | oxycodone hydrochloride |
| 0054-0393 | Oxycodone Hydrochloride | Oxycodone Hydrochloride |
| 0054-0522 | Oxycodone Hydrochloride | Oxycodone Hydrochloride |
| 0093-5731 | Oxycodone Hydrochloride | Oxycodone Hydrochloride |
| 0093-5732 | Oxycodone Hydrochloride | Oxycodone Hydrochloride |
| 0093-5733 | Oxycodone Hydrochloride | Oxycodone Hydrochloride |
| 0093-5734 | Oxycodone Hydrochloride | Oxycodone Hydrochloride |
| 0115-1556 | OXYCODONE HYDROCHLORIDE | OXYCODONE HYDROCHLORIDE |
| 0115-1557 | OXYCODONE HYDROCHLORIDE | OXYCODONE HYDROCHLORIDE |
| 0115-1558 | OXYCODONE HYDROCHLORIDE | OXYCODONE HYDROCHLORIDE |
| 0115-1559 | OXYCODONE HYDROCHLORIDE | OXYCODONE HYDROCHLORIDE |
Trademark Results [OxyContin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OXYCONTIN 90470025 not registered Live/Pending |
Yangzhou Zhixing Network Technology Co., Ltd. 2021-01-15 |
 OXYCONTIN 75069553 2033914 Live/Registered |
PURDUE PHARMA L.P. 1996-03-08 |
 OXYCONTIN 74339006 2004586 Live/Registered |
PURDUE PHARMA L.P. 1992-12-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.