NDC 22840-1515
Red Clover
Trifolium Pratense
Red Clover is a Intradermal; Percutaneous; Subcutaneous Solution in the Non-standardized Allergenic category. It is labeled and distributed by Greer Laboratories, Inc.. The primary component is Trifolium Pratense Pollen.
| Product ID | 22840-1515_7a2bec20-8dae-e536-e053-2a91aa0ab928 |
| NDC | 22840-1515 |
| Product Type | Non-standardized Allergenic |
| Proprietary Name | Red Clover |
| Generic Name | Trifolium Pratense |
| Dosage Form | Solution |
| Route of Administration | INTRADERMAL; PERCUTANEOUS; SUBCUTANEOUS |
| Marketing Start Date | 1981-09-15 |
| Marketing Category | BLA / BLA |
| Application Number | BLA101833 |
| Labeler Name | Greer Laboratories, Inc. |
| Substance Name | TRIFOLIUM PRATENSE POLLEN |
| Active Ingredient Strength | 0 g/mL |
| Pharm Classes | Increased Histamine Release [PE],Cell-mediated Immunity [PE],Increased IgG Production [PE],Pollen [CS],Allergens [CS],Non-Standardized Pollen Allergenic Extract [EPC] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 22840-1515-1
5 mL in 1 VIAL, MULTI-DOSE (22840-1515-1)
| Marketing Start Date | 1981-09-15 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 22840-1515-1 [22840151501]
Red Clover SOLUTION
| Marketing Category | BLA |
| Application Number | BLA101833 |
| Product Type | NON-STANDARDIZED ALLERGENIC |
| Marketing Start Date | 1981-09-15 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| TRIFOLIUM PRATENSE POLLEN | .001 g/mL |
Pharmacological Class
- Increased Histamine Release [PE]
- Cell-mediated Immunity [PE]
- Increased IgG Production [PE]
- Pollen [CS]
- Allergens [CS]
- Non-Standardized Pollen Allergenic Extract [EPC]
NDC Crossover Matching brand name "Red Clover" or generic name "Trifolium Pratense"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 22840-1514 | Red Clover | Trifolium pratense |
| 22840-1515 | Red Clover | Trifolium pratense |
| 22840-1565 | Red Clover | Trifolium pratense |
| 22840-5506 | Red Clover | Trifolium pratense |
| 15631-0446 | TRIFOLIUM PRATENSE | TRIFOLIUM PRATENSE |
Trademark Results [Red Clover]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
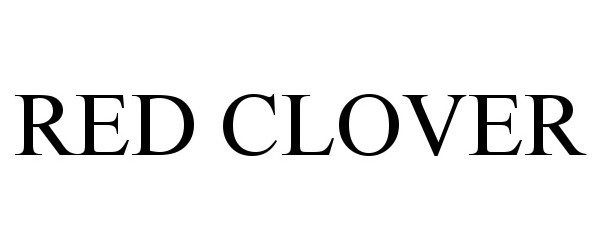 RED CLOVER 97077082 not registered Live/Pending |
Red Clover, Inc. 2021-10-15 |
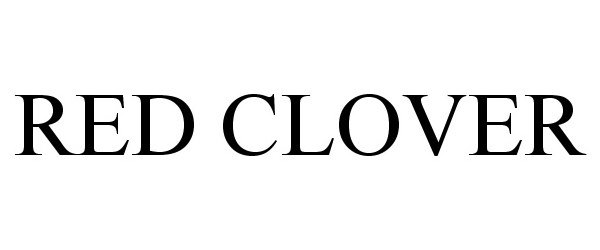 RED CLOVER 87297524 5325699 Live/Registered |
RED CLOVER, LLC 2017-01-11 |
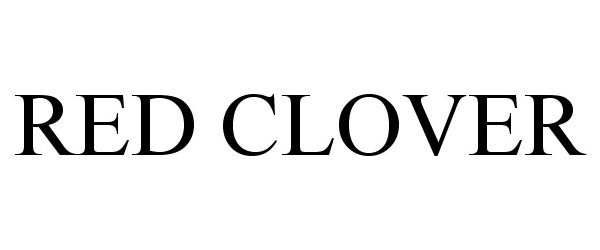 RED CLOVER 86915957 not registered Dead/Abandoned |
Red Clover, LLC 2016-02-22 |
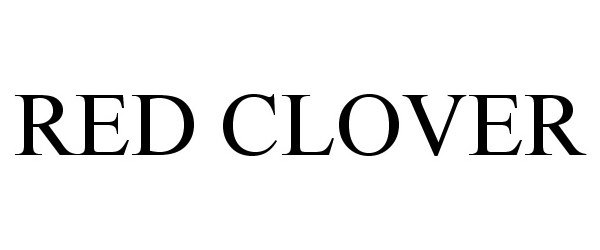 RED CLOVER 85622986 not registered Dead/Abandoned |
LUCKY BRAND DUNGAREES, LLC 2012-05-11 |
 RED CLOVER 85622981 not registered Dead/Abandoned |
Lucky Brand Dungarees, LLC 2012-05-11 |
 RED CLOVER 85622975 not registered Dead/Abandoned |
Lucky Brand Dungarees, LLC 2012-05-11 |
 RED CLOVER 85354827 not registered Dead/Abandoned |
Lucky Brand Dungarees, Inc. 2011-06-23 |
 RED CLOVER 85354821 not registered Dead/Abandoned |
Lucky Brand Dungarees, Inc. 2011-06-23 |
 RED CLOVER 85354813 not registered Dead/Abandoned |
Lucky Brand Dungarees, Inc. 2011-06-23 |
 RED CLOVER 85135748 not registered Dead/Abandoned |
Aalfs Manufacturing, Inc. 2010-09-22 |
 RED CLOVER 74489916 1887348 Dead/Cancelled |
PILLSBURY COMPANY, THE 1994-02-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.