NDC 29485-6647
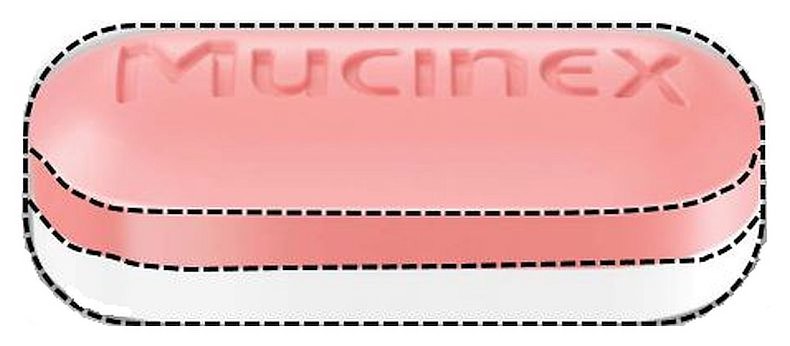
Mucinex DM
Dextromethorphan Hydrobromide And Guaifenesin
Mucinex DM is a Oral Tablet, Extended Release in the Human Otc Drug category. It is labeled and distributed by Mechanical Servants Llc. The primary component is Dextromethorphan Hydrobromide; Guaifenesin.
| Product ID | 29485-6647_b4403eee-ae25-9c42-e053-2995a90a49cb |
| NDC | 29485-6647 |
| Product Type | Human Otc Drug |
| Proprietary Name | Mucinex DM |
| Generic Name | Dextromethorphan Hydrobromide And Guaifenesin |
| Dosage Form | Tablet, Extended Release |
| Route of Administration | ORAL |
| Marketing Start Date | 2012-06-26 |
| Marketing Category | NDA / NDA |
| Application Number | NDA021620 |
| Labeler Name | Mechanical Servants LLC |
| Substance Name | DEXTROMETHORPHAN HYDROBROMIDE; GUAIFENESIN |
| Active Ingredient Strength | 30 mg/1; mg/1 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2021-12-31 |
Packaging
NDC 29485-6647-2
2 TABLET, EXTENDED RELEASE in 1 POUCH (29485-6647-2)
| Marketing Start Date | 2012-06-26 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 29485-6647-2 [29485664702]
Mucinex DM TABLET, EXTENDED RELEASE
| Marketing Category | NDA |
| Application Number | NDA021620 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2012-06-26 |
| Inactivation Date | 2020-01-31 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| DEXTROMETHORPHAN HYDROBROMIDE | 30 mg/1 |
OpenFDA Data
| SPL SET ID: | 0fc54199-9a61-4a63-b5cd-b132e44f23c9 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
NDC Crossover Matching brand name "Mucinex DM" or generic name "Dextromethorphan Hydrobromide And Guaifenesin"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 17856-0056 | Mucinex DM | Guaifenesin and Dextromethorphan Hydrobromide |
| 67751-171 | Mucinex DM | Mucinex DM |
| 68788-6340 | Mucinex DM | Mucinex DM |
| 50090-1078 | Mucinex DM | Mucinex DM |
| 50090-1077 | Mucinex DM | Mucinex DM |
| 50090-2299 | Mucinex DM | Mucinex DM |
| 63824-056 | Mucinex DM | Mucinex DM |
| 66715-9724 | Mucinex DM | Mucinex DM |
| 0031-8702 | Childrens Robitussin Cough and Chest Congestion DM | dextromethorphan hydrobromide and guaifenesin |
| 11523-7158 | Coricidin HBP Chest Congestion And Cough | Dextromethorphan hydrobromide and Guaifenesin |
| 0363-0115 | MAXIMUM STRENGTH COUGH PLUS CHEST CONGESTION DM | Dextromethorphan Hydrobromide and Guaifenesin |
| 49035-839 | Maximum Strength Mucus Relief DM | Dextromethorphan hydrobromide and Guaifenesin |
| 29485-6647 | Mucinex | DEXTROMETHORPHAN HYDROBROMIDE and GUAIFENESIN |
| 29485-6942 | Mucinex | DEXTROMETHORPHAN HYDROBROMIDE and GUAIFENESIN |
| 11673-109 | Mucus and Cough Relief | Dextromethorphan Hydrobromide and Guaifenesin |
| 49738-109 | Mucus DM | Dextromethorphan Hydrobromide and Guaifenesin |
| 30142-109 | Mucus Relief DM | Dextromethorphan Hydrobromide and Guaifenesin |
| 0363-6005 | Mucus Relief DM Honey and Berry Flavor | Dextromethorphan Hydrobromide and Guaifenesin |
| 0031-8757 | ROBITUSSIN COUGH PLUS CHEST CONGESTION DM | dextromethorphan hydrobromide and guaifenesin |
| 0031-8719 | Robitussin Maximum Strength Cough Plus Chest Congestion DM | dextromethorphan hydrobromide and guaifenesin |
| 0031-8736 | Robitussin Peak Cold Cough Plus Chest Congestion DM | dextromethorphan hydrobromide and guaifenesin |
| 0031-8737 | Robitussin Peak Cold Sugar-Free Cough Plus Chest Congestion DM | dextromethorphan hydrobromide and guaifenesin |
| 15187-050 | Scot-Tussin Senior Maximum Strength Cough Suppressant and Expectorant | Dextromethorphan Hydrobromide and Guaifenesin |
| 37000-510 | Vicks DayQuil | Dextromethorphan Hydrobromide and Guaifenesin |
| 0363-7296 | Walgreen | Dextromethorphan Hydrobromide and Guaifenesin |
| 0363-7440 | WALGREEN ADULT COUGH PLUS CHEST CONGESTION DM | dextromethorphan hydrobromide and guaifenesin |
Trademark Results [Mucinex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MUCINEX 98499792 not registered Live/Pending |
RB Health (US) LLC 2024-04-15 |
 MUCINEX 90229382 not registered Live/Pending |
RB Health (US) LLC 2020-10-01 |
 MUCINEX 88040437 not registered Live/Pending |
Reckitt Benckiser LLC 2018-07-17 |
 MUCINEX 86191608 4613098 Live/Registered |
RB HEALTH (US) LLC 2014-02-12 |
 MUCINEX 85496330 4168070 Live/Registered |
RB HEALTH (US) LLC 2011-12-15 |
 MUCINEX 85489681 4168052 Live/Registered |
RB HEALTH (US) LLC 2011-12-07 |
 MUCINEX 77770135 3722363 Live/Registered |
Reckitt Benckiser Inc. 2009-06-29 |
 MUCINEX 76297961 2670161 Live/Registered |
RB HEALTH (US) LLC 2001-08-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.