NDC 40104-556
Virx
Instant Hand Sanitizing Spray With Aloe Vera
Virx is a Topical Spray in the Human Otc Drug category. It is labeled and distributed by Ningbo Pulisi Daily Chemical Products Co., Ltd. The primary component is Triclosan.
| Product ID | 40104-556_9f62a572-23d1-409d-859e-711ee23e7aef |
| NDC | 40104-556 |
| Product Type | Human Otc Drug |
| Proprietary Name | Virx |
| Generic Name | Instant Hand Sanitizing Spray With Aloe Vera |
| Dosage Form | Spray |
| Route of Administration | TOPICAL |
| Marketing Start Date | 2009-09-30 |
| Marketing Category | OTC MONOGRAPH FINAL / OTC MONOGRAPH FINAL |
| Application Number | part333 |
| Labeler Name | Ningbo Pulisi Daily Chemical Products Co., Ltd |
| Substance Name | TRICLOSAN |
| Active Ingredient Strength | 1 mL/10mL |
| NDC Exclude Flag | E |
| Listing Certified Through | 2017-12-31 |
Packaging
NDC 40104-556-06
1 TUBE in 1 PACKAGE (40104-556-06) > 10 mL in 1 TUBE
| Marketing Start Date | 2009-09-30 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 40104-556-06 [40104055606]
Virx SPRAY
| Marketing Category | OTC monograph final |
| Application Number | part333 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2009-09-30 |
| Inactivation Date | 2019-10-21 |
NDC 40104-556-08 [40104055608]
Virx SPRAY
| Marketing Category | OTC monograph final |
| Application Number | part333 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2009-09-30 |
| Inactivation Date | 2019-10-21 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| TRICLOSAN | 1.2 mL/10mL |
OpenFDA Data
| SPL SET ID: | f543d58a-ab11-49dc-985a-b506f7838bf2 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI | |
| UPC Code |
NDC Crossover Matching brand name "Virx" or generic name "Instant Hand Sanitizing Spray With Aloe Vera"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 29860-200 | Virx | Instant Hand Sanitizer |
| 29860-203 | Virx | Instant Hand Sanitizer with Security Clip |
| 29860-205 | Virx | Instant Hand Sanitizer |
| 40104-556 | Virx | Instant Hand Sanitizing Spray with Aloe Vera |
| 40104-703 | Virx | Instant Hand Sanitizer |
| 40104-811 | Virx | Hand Sanitizer and Moisturizer with Shea Butter |
| 50438-807 | Virx | Instant Hand Sanitizer |
Trademark Results [Virx]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
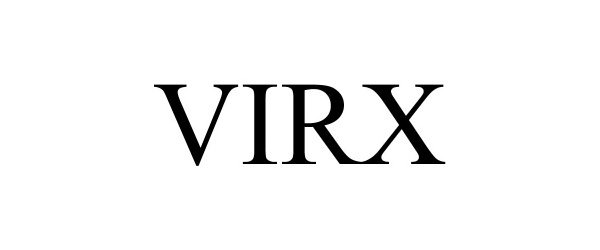 VIRX 97053535 not registered Live/Pending |
Oven Industries, Inc. 2021-09-30 |
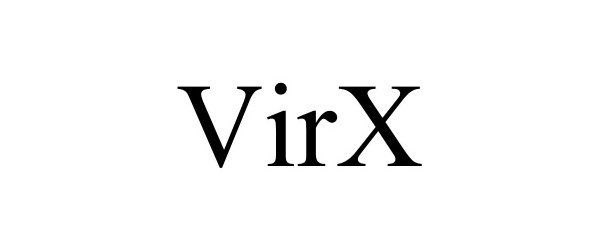 VIRX 90840548 not registered Live/Pending |
SaNOtize Research and Development Corp. 2021-07-21 |
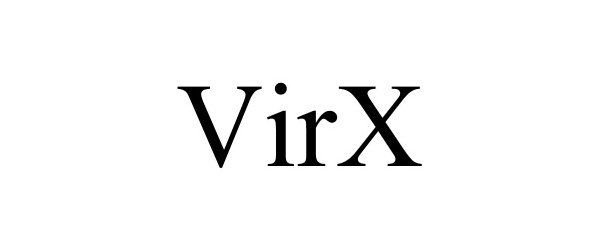 VIRX 90494079 not registered Live/Pending |
HMI Industries, Inc. 2021-01-28 |
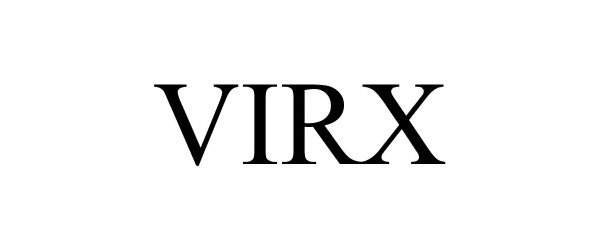 VIRX 88503688 not registered Live/Pending |
FILO AMERICA 2019-07-08 |
 VIRX 86526109 not registered Dead/Abandoned |
MedForce Solutions, Inc. 2015-02-05 |
 VIRX 77230009 3397984 Dead/Cancelled |
Filo America 2007-07-15 |
 VIRX 75561284 not registered Dead/Abandoned |
Gel Tech Industries, Inc. 1998-09-29 |
 VIRX 73741458 1563908 Dead/Cancelled |
VIRX, INC. 1988-07-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.