NDC 42367-531
Pemfexy
Pemetrexed
Pemfexy is a Intravenous Injection in the Human Prescription Drug category. It is labeled and distributed by Eagle Pharmaceuticals, Inc.. The primary component is Pemetrexed Monohydrate.
| Product ID | 42367-531_543ac453-8dd6-45e3-b0f1-07f776ad2325 |
| NDC | 42367-531 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Pemfexy |
| Generic Name | Pemetrexed |
| Dosage Form | Injection |
| Route of Administration | INTRAVENOUS |
| Marketing Start Date | 2022-02-01 |
| Marketing Category | NDA / |
| Application Number | NDA209472 |
| Labeler Name | Eagle Pharmaceuticals, Inc. |
| Substance Name | PEMETREXED MONOHYDRATE |
| Active Ingredient Strength | 25 mg/mL |
| Pharm Classes | Folate Analog Metabolic Inhibitor [EPC], Folic Acid Metabolism Inhibitors [MoA] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2023-12-31 |
Packaging
NDC 42367-531-33
1 VIAL in 1 CARTON (42367-531-33) > 20 mL in 1 VIAL
| Marketing Start Date | 2022-02-01 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "Pemfexy" or generic name "Pemetrexed"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 42367-531 | Pemfexy | pemetrexed |
| 0338-0720 | PEMETREXED | PEMETREXED |
| 0338-0722 | PEMETREXED | PEMETREXED |
| 0409-1045 | PEMETREXED | PEMETREXED |
| 0409-1060 | Pemetrexed | Pemetrexed |
| 0409-1061 | Pemetrexed | Pemetrexed |
| 0409-1062 | Pemetrexed | Pemetrexed |
| 0409-2188 | PEMETREXED | PEMETREXED |
| 0409-3532 | PEMETREXED | PEMETREXED |
| 0480-4514 | Pemetrexed | Pemetrexed |
| 0480-4515 | Pemetrexed | Pemetrexed |
| 0480-4516 | Pemetrexed | Pemetrexed |
| 16729-229 | Pemetrexed | Pemetrexed |
| 16729-230 | Pemetrexed | Pemetrexed |
| 16729-244 | Pemetrexed | Pemetrexed |
| 55150-381 | Pemetrexed | Pemetrexed |
| 55150-382 | Pemetrexed | Pemetrexed |
| 55150-383 | Pemetrexed | Pemetrexed |
| 68001-535 | Pemetrexed | Pemetrexed |
Trademark Results [Pemfexy]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
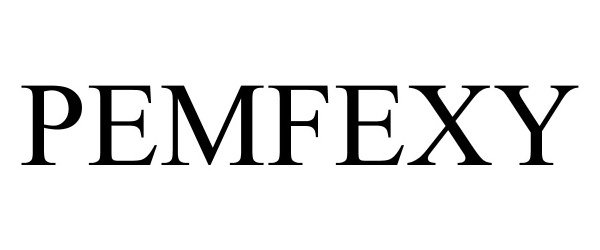 PEMFEXY 90182483 not registered Live/Pending |
Eagle Pharmaceuticals Inc. 2020-09-15 |
 PEMFEXY 88512003 not registered Live/Pending |
Eagle Pharmaceuticals Inc. 2019-07-12 |
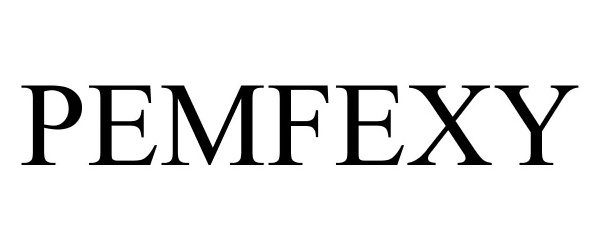 PEMFEXY 87365311 not registered Live/Pending |
Eagle Pharmaceuticals Inc. 2017-03-09 |
 PEMFEXY 87365309 not registered Live/Pending |
Eagle Pharmaceuticals Inc. 2017-03-09 |
 PEMFEXY 87365305 not registered Live/Pending |
Eagle Pharmaceuticals Inc. 2017-03-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.