NDC 49781-101
All Day Pain Relief
Naproxen Sodium
All Day Pain Relief is a Oral Tablet in the Human Otc Drug category. It is labeled and distributed by Cardinal Health (leader) 49781. The primary component is Naproxen Sodium.
| Product ID | 49781-101_37be6747-1ad2-405b-9e55-67e4b91c0626 |
| NDC | 49781-101 |
| Product Type | Human Otc Drug |
| Proprietary Name | All Day Pain Relief |
| Generic Name | Naproxen Sodium |
| Dosage Form | Tablet |
| Route of Administration | ORAL |
| Marketing Start Date | 2014-07-31 |
| Marketing Category | ANDA / ANDA |
| Application Number | ANDA091353 |
| Labeler Name | Cardinal Health (Leader) 49781 |
| Substance Name | NAPROXEN SODIUM |
| Active Ingredient Strength | 220 mg/1 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 49781-101-51
1 BOTTLE, PLASTIC in 1 BOX (49781-101-51) > 100 TABLET in 1 BOTTLE, PLASTIC
| Marketing Start Date | 2014-07-31 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 49781-101-50 [49781010150]
All Day Pain Relief TABLET
| Marketing Category | ANDA |
| Application Number | ANDA091353 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2014-07-31 |
| Marketing End Date | 2020-07-31 |
NDC 49781-101-56 [49781010156]
All Day Pain Relief TABLET
| Marketing Category | ANDA |
| Application Number | ANDA091353 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2014-07-31 |
| Marketing End Date | 2020-07-31 |
NDC 49781-101-51 [49781010151]
All Day Pain Relief TABLET
| Marketing Category | ANDA |
| Application Number | ANDA091353 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2014-07-31 |
| Marketing End Date | 2020-07-31 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| NAPROXEN SODIUM | 220 mg/1 |
OpenFDA Data
| SPL SET ID: | f174c5c8-eaca-4306-a65e-8f13b9488da3 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI | |
| UPC Code |
NDC Crossover Matching brand name "All Day Pain Relief" or generic name "Naproxen Sodium"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 68210-0030 | ALL DAY PAIN RELIEF | ALL DAY PAIN RELIEF |
| 69517-109 | All Day Pain Relief | All Day Pain Relief |
| 70005-008 | All Day Pain Relief | All Day Pain Relief |
| 15127-168 | All Day Pain Relief | All Day Pain Relief |
| 30142-168 | All Day Pain Relief | All Day Pain Relief |
| 36800-256 | All Day Pain Relief | All Day Pain Relief |
| 37808-259 | All Day Pain Relief | All Day Pain Relief |
| 37205-744 | All Day Pain Relief | All Day Pain Relief |
| 37808-068 | All Day Pain Relief | All Day Pain Relief |
| 37808-339 | all day pain relief | all day pain relief |
| 37808-500 | All Day Pain Relief | All Day Pain Relief |
| 41163-167 | All Day Pain Relief | All Day Pain Relief |
| 41163-169 | All Day Pain Relief | All Day Pain Relief |
| 42254-230 | all day pain relief | all day pain relief |
| 49781-145 | All Day Pain Relief | All Day Pain Relief |
| 49781-101 | All Day Pain Relief | All Day Pain Relief |
| 49781-046 | All Day Pain Relief | All Day Pain Relief |
| 49781-146 | All Day Pain Relief | All Day Pain Relief |
| 49781-047 | All Day Pain Relief | All Day Pain Relief |
| 55315-168 | All Day Pain Relief | All Day Pain Relief |
| 55315-256 | All Day Pain Relief | All Day Pain Relief |
| 55315-255 | All Day Pain Relief | All Day Pain Relief |
| 55315-169 | All Day Pain Relief | All Day Pain Relief |
| 55319-417 | All Day Pain Relief | All Day Pain Relief |
| 55319-604 | All Day Pain Relief | All Day Pain Relief |
| 55319-174 | All Day Pain Relief | All Day Pain Relief |
| 59726-169 | All Day Pain Relief | All Day Pain Relief |
| 59779-172 | All Day Pain Relief | All Day Pain Relief |
| 59726-023 | All Day Pain Relief | All Day Pain Relief |
| 59726-168 | All Day Pain Relief | All Day Pain Relief |
| 0363-0169 | All Day Pain Relief | All Day Pain Relief |
| 59726-256 | All Day Pain Relief | All Day Pain Relief |
| 0363-0168 | All Day Pain Relief | All Day Pain Relief |
| 0363-0368 | All Day Pain Relief | All Day Pain Relief |
| 0363-0938 | all day pain relief | all day pain relief |
| 63940-174 | All Day Pain Relief | All Day Pain Relief |
| 0363-6099 | ALL DAY PAIN RELIEF | ALL DAY PAIN RELIEF |
| 0363-9608 | ALL DAY PAIN RELIEF | ALL DAY PAIN RELIEF |
| 0280-6000 | Aleve | NAPROXEN SODIUM |
| 0280-6010 | Aleve | NAPROXEN SODIUM |
| 0280-6020 | Aleve | NAPROXEN SODIUM |
| 0280-0041 | Aleve Headache Pain | Naproxen Sodium |
| 0113-7033 | basic care naproxen sodium | Naproxen Sodium |
| 0113-7368 | Basic Care Naproxen Sodium | Naproxen Sodium |
| 0113-7901 | basic care naproxen sodium | Naproxen Sodium |
| 0113-0901 | Good Sense Naproxen Sodium | Naproxen Sodium |
| 0113-1412 | good sense naproxen sodium | naproxen sodium |
| 0113-1773 | good sense naproxen sodium | Naproxen Sodium |
| 0113-4368 | Good Sense Naproxen Sodium | Naproxen Sodium |
| 0280-0270 | Menstridol | NAPROXEN SODIUM |
Trademark Results [All Day]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
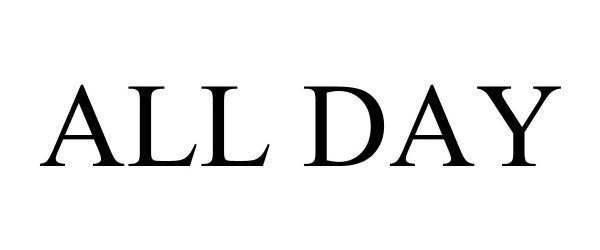 ALL DAY 98076158 not registered Live/Pending |
Zachariah James Leonard 2023-07-08 |
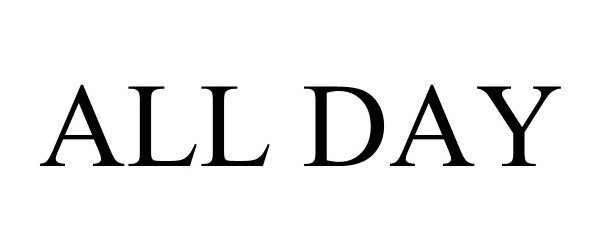 ALL DAY 97916400 not registered Live/Pending |
Canal Street Brewing Company, LLC 2023-05-02 |
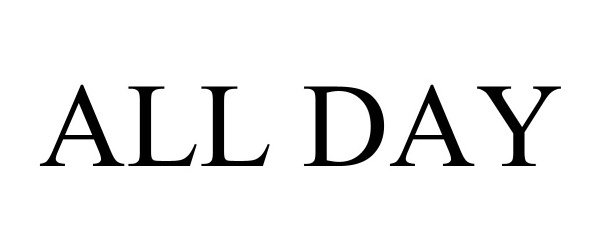 ALL DAY 97752677 not registered Live/Pending |
All Day Coffee & Eggs LLC 2023-01-12 |
 ALL DAY 97203923 not registered Live/Pending |
Starday Foods, Inc. 2022-01-05 |
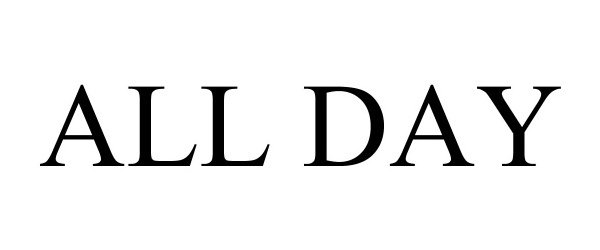 ALL DAY 97087791 not registered Live/Pending |
Dapper Labs Inc. 2021-10-22 |
 ALL DAY 97087784 not registered Live/Pending |
Dapper Labs Inc. 2021-10-22 |
 ALL DAY 97087772 not registered Live/Pending |
Dapper Labs Inc. 2021-10-22 |
 ALL DAY 97087770 not registered Live/Pending |
Dapper Labs Inc. 2021-10-22 |
 ALL DAY 97087763 not registered Live/Pending |
Dapper Labs Inc. 2021-10-22 |
 ALL DAY 90743413 not registered Live/Pending |
All Day Spirits Corp. 2021-05-29 |
 ALL DAY 90534957 not registered Live/Pending |
All Day LLC 2021-02-18 |
 ALL DAY 87079437 not registered Dead/Abandoned |
General Resources LLC 2016-06-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.