NDC 51393-4012
Wrinkle Control
Thuja Occidentalis Leaf
Wrinkle Control is a Topical Solution/ Drops in the Human Otc Drug category. It is labeled and distributed by Forces Of Nature. The primary component is Thuja Occidentalis Leaf.
| Product ID | 51393-4012_089852c9-4482-45d0-a85d-d4fa7d0a79fe |
| NDC | 51393-4012 |
| Product Type | Human Otc Drug |
| Proprietary Name | Wrinkle Control |
| Generic Name | Thuja Occidentalis Leaf |
| Dosage Form | Solution/ Drops |
| Route of Administration | TOPICAL |
| Marketing Start Date | 2013-03-15 |
| Marketing Category | UNAPPROVED HOMEOPATHIC / UNAPPROVED HOMEOPATHIC |
| Labeler Name | Forces of Nature |
| Substance Name | THUJA OCCIDENTALIS LEAF |
| Active Ingredient Strength | 6 [hp_X]/100mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 51393-4012-2
33 mL in 1 BOTTLE, DISPENSING (51393-4012-2)
| Marketing Start Date | 2013-03-15 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 51393-4012-2 [51393401202]
Wrinkle Control SOLUTION/ DROPS
| Marketing Category | UNAPPROVED HOMEOPATHIC |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2013-03-15 |
| Inactivation Date | 2020-01-31 |
NDC 51393-4012-1 [51393401201]
Wrinkle Control SOLUTION/ DROPS
| Marketing Category | UNAPPROVED HOMEOPATHIC |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2013-03-15 |
| Inactivation Date | 2020-01-31 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| THUJA OCCIDENTALIS LEAF | 6 [hp_X]/100mL |
OpenFDA Data
| SPL SET ID: | afd92705-bd32-48f6-a322-7f44df41b973 |
| Manufacturer | |
| UNII |
NDC Crossover Matching brand name "Wrinkle Control" or generic name "Thuja Occidentalis Leaf"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 51393-4012 | Wrinkle Control | THUJA OCCIDENTALIS LEAF |
| 51393-4007 | Scabies Control | THUJA OCCIDENTALIS LEAF |
Trademark Results [Wrinkle Control]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
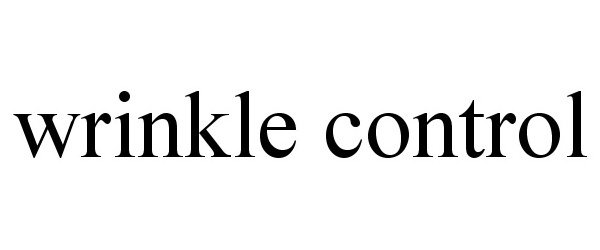 WRINKLE CONTROL 77775212 3795260 Dead/Cancelled |
pac fung feather co., ltd. 2009-07-07 |
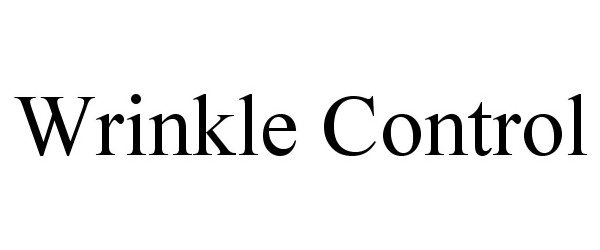 WRINKLE CONTROL 77412504 not registered Dead/Abandoned |
Heuer, Marvin A. 2008-03-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.