NDC 51862-471
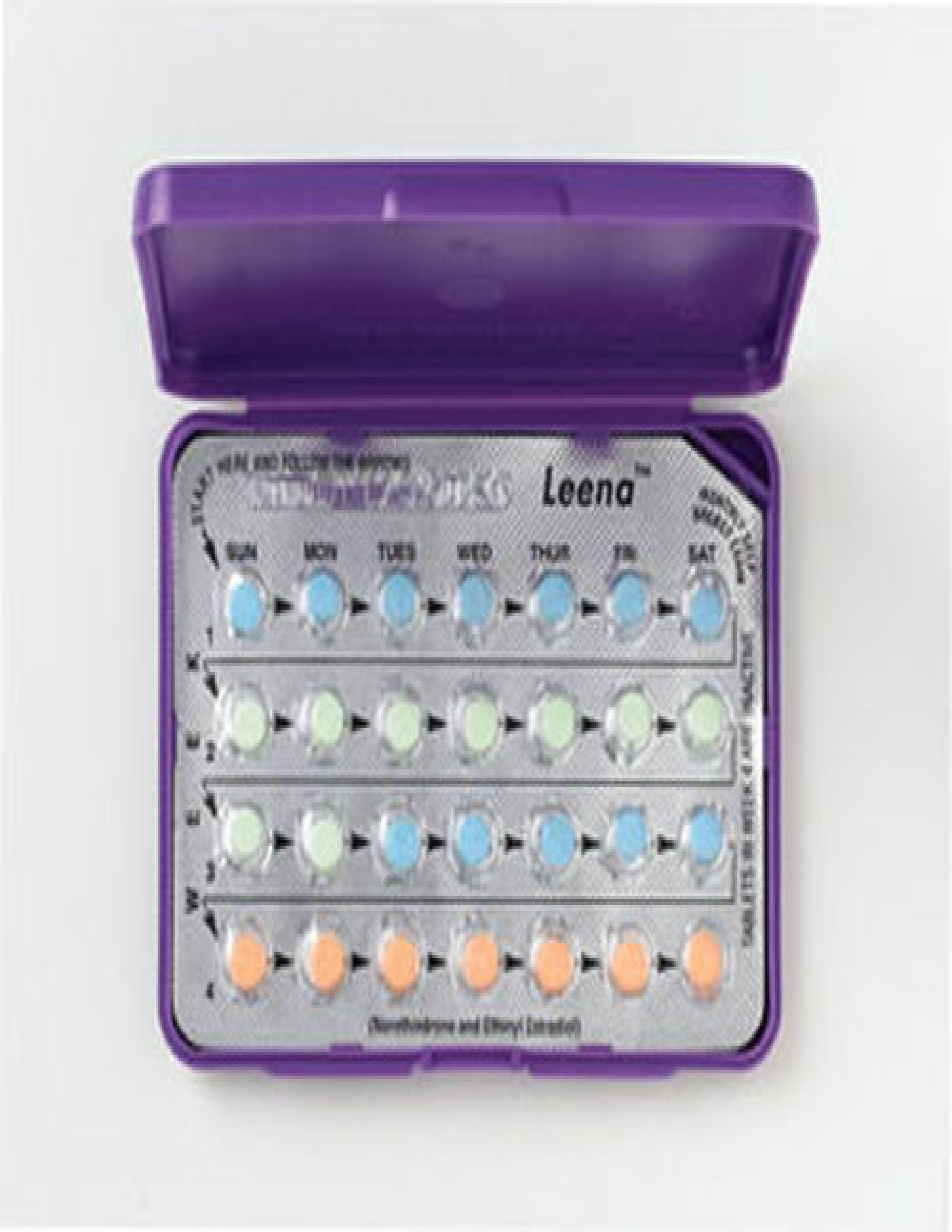
Leena
Norethindrone And Ethinyl Estradiol
Leena is a Kit in the Human Prescription Drug category. It is labeled and distributed by Mayne Pharma Inc.. The primary component is .
| Product ID | 51862-471_4280194b-de76-465d-9e86-8563fbf81301 |
| NDC | 51862-471 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Leena |
| Generic Name | Norethindrone And Ethinyl Estradiol |
| Dosage Form | Kit |
| Marketing Start Date | 2016-08-03 |
| Marketing Category | NDA AUTHORIZED GENERIC / NDA AUTHORIZED GENERIC |
| Application Number | NDA018977 |
| Labeler Name | Mayne Pharma Inc. |
| Active Ingredient Strength | 0 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2023-12-31 |
Packaging
NDC 51862-471-06
6 BLISTER PACK in 1 CARTON (51862-471-06) > 1 KIT in 1 BLISTER PACK (51862-471-01)
| Marketing Start Date | 2016-08-03 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 51862-471-06 [51862047106]
Leena KIT
| Marketing Category | NDA authorized generic |
| Application Number | NDA018977 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2016-08-03 |
NDC 51862-471-01 [51862047101]
Leena KIT
| Marketing Category | NDA authorized generic |
| Application Number | NDA018977 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2016-09-06 |
Drug Details
OpenFDA Data
| SPL SET ID: | 123a7d41-f39f-421d-aa0e-2d36cc312c46 |
| Manufacturer | |
| RxNorm Concept Unique ID - RxCUI |
Medicade Reported Pricing
51862047106 LEENA 28 TABLET
Pricing Unit: EA | Drug Type:
51862047101 LEENA 28 TABLET
Pricing Unit: EA | Drug Type:
NDC Crossover Matching brand name "Leena" or generic name "Norethindrone And Ethinyl Estradiol"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 51862-471 | Leena | Leena |
| 50090-1477 | ALYACEN 1/35 | norethindrone and ethinyl estradiol |
| 50090-1478 | ALYACEN 7/7/7 | norethindrone and ethinyl estradiol |
| 0555-9066 | Aranelle | norethindrone and ethinyl estradiol |
| 0555-9034 | Balziva | Norethindrone and Ethinyl Estradiol |
| 0603-7521 | Cyclafem 1/35 | norethindrone and ethinyl estradiol |
| 0603-7525 | Cyclafem 7/7/7 | norethindrone and ethinyl estradiol |
| 16714-348 | Dasetta 1/35 | Norethindrone and Ethinyl Estradiol |
| 16714-346 | Dasetta 7/7/7 | norethindrone and ethinyl estradiol |
| 21695-857 | Necon | Norethindrone and Ethinyl Estradiol |
| 0093-3303 | Necon 1/35 | Norethindrone and Ethinyl Estradiol |
| 0555-9008 | Nortrel | Norethindrone and Ethinyl Estradiol |
| 0555-9009 | Nortrel | Norethindrone and Ethinyl Estradiol |
| 0555-9010 | Nortrel | Norethindrone and Ethinyl Estradiol |
| 0555-9012 | Nortrel 7/7/7 | Norethindrone and Ethinyl Estradiol |
| 50090-0159 | Ortho-Novum | norethindrone and ethinyl estradiol |
| 16714-370 | Wera | norethindrone and ethinyl estradiol |
| 50090-1431 | Wera | Norethindrone and Ethinyl Estradiol |
Trademark Results [Leena]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LEENA 76505310 2998666 Live/Registered |
MAYNE PHARMA LLC 2003-04-09 |
 LEENA 74120625 not registered Dead/Abandoned |
Wings Manufacturing Corporation 1990-12-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.