NDC 53063-1113
GO TIME
Ammonia
GO TIME is a Respiratory (inhalation) Inhalant in the Human Otc Drug category. It is labeled and distributed by Mountain Top Labs, Llc. The primary component is Ammonia.
| Product ID | 53063-1113_ae3e24ae-ab8b-46af-a0db-1ba9a3802bc6 |
| NDC | 53063-1113 |
| Product Type | Human Otc Drug |
| Proprietary Name | GO TIME |
| Generic Name | Ammonia |
| Dosage Form | Inhalant |
| Route of Administration | RESPIRATORY (INHALATION) |
| Marketing Start Date | 2012-08-13 |
| Marketing Category | UNAPPROVED DRUG OTHER / UNAPPROVED DRUG OTHER |
| Labeler Name | Mountain Top Labs, LLC |
| Substance Name | AMMONIA |
| Active Ingredient Strength | 0 mL/.3mL |
| NDC Exclude Flag | E |
| Listing Certified Through | 2017-12-31 |
Packaging
NDC 53063-1113-2
4 AMPULE in 1 BOX (53063-1113-2) > .3 mL in 1 AMPULE (53063-1113-1)
| Marketing Start Date | 2012-08-13 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 53063-1113-2 [53063111302]
GO TIME INHALANT
| Marketing Category | unapproved drug other |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2012-08-13 |
| Inactivation Date | 2020-01-31 |
NDC 53063-1113-1 [53063111301]
GO TIME INHALANT
| Marketing Category | unapproved drug other |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2012-08-13 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| AMMONIA | .013 mL/.3mL |
OpenFDA Data
| SPL SET ID: | bd4b2cc4-b9ed-4415-b9a4-5fdff8aa292d |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
NDC Crossover Matching brand name "GO TIME" or generic name "Ammonia"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 53063-1111 | GO TIME | Ammonia |
| 53063-1112 | GO TIME | Ammonia |
| 53063-1113 | GO TIME | Ammonia |
| 53063-1114 | GO TIME | Ammonia |
| 55670-710 | Ammonia Inhalant | Ammonia |
| 73395-196 | Ammonia Inhalant | Ammonia |
| 50332-0054 | Ammonia Inhalants | ammonia |
| 52124-3201 | Ammonia Pad | AMMONIA |
| 68428-878 | Ammonium causticum | AMMONIA |
| 71919-042 | Ammonium causticum | AMMONIA |
| 69842-407 | CVS Health First Aid Outdoor Prep-Pack | Ammonia |
Trademark Results [GO TIME]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GO TIME 97223138 not registered Live/Pending |
Davis, Richard 2022-01-17 |
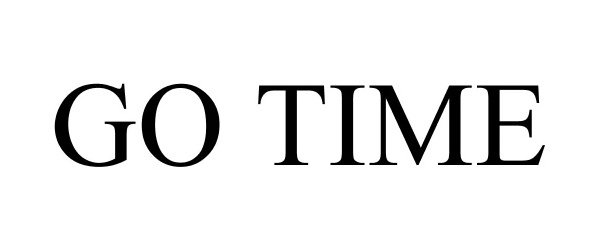 GO TIME 88431106 not registered Live/Pending |
Bridgestone Brands, LLC 2019-05-15 |
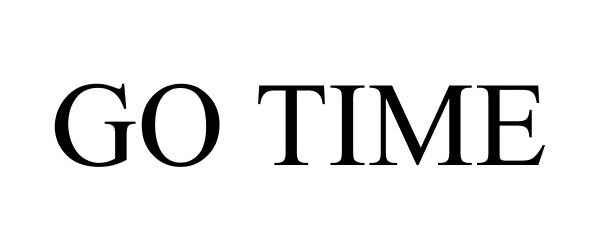 GO TIME 87236853 5382011 Live/Registered |
Young Nails, Inc. 2016-11-15 |
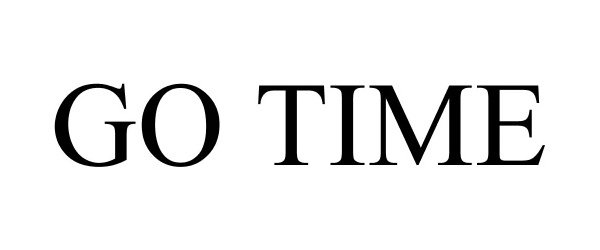 GO TIME 85548863 4343560 Dead/Cancelled |
Mountain Top Labs, LLC 2012-02-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.