NDC 58160-815
TWINRIX
Hepatitis A And Hepatitis B (recombinant) Vaccine
TWINRIX is a Intramuscular Injection, Suspension in the Vaccine category. It is labeled and distributed by Glaxosmithkline Biologicals Sa. The primary component is Hepatitis A Virus Strain Hm175 Antigen (formaldehyde Inactivated); Hepatitis B Virus Subtype Adw2 Hbsag Surface Protein Antigen.
| Product ID | 58160-815_169a9d49-e1fe-40eb-bc20-c03907c02c73 |
| NDC | 58160-815 |
| Product Type | Vaccine |
| Proprietary Name | TWINRIX |
| Generic Name | Hepatitis A And Hepatitis B (recombinant) Vaccine |
| Dosage Form | Injection, Suspension |
| Route of Administration | INTRAMUSCULAR |
| Marketing Start Date | 2007-06-07 |
| Marketing Category | BLA / BLA |
| Application Number | BLA103850 |
| Labeler Name | GlaxoSmithKline Biologicals SA |
| Substance Name | HEPATITIS A VIRUS STRAIN HM175 ANTIGEN (FORMALDEHYDE INACTIVATED); HEPATITIS B VIRUS SUBTYPE ADW2 HBSAG SURFACE PROTEIN ANTIGEN |
| Active Ingredient Strength | 720 [iU]/mL; ug/mL |
| Pharm Classes | Inactivated Hepatitis A Virus Vaccine [EPC],Actively Acquired Immunity [PE],Hepatitis A Vaccines [CS],Vaccines, Inactivated [CS] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2020-12-31 |
Packaging
NDC 58160-815-52
10 SYRINGE in 1 CARTON (58160-815-52) > 1 mL in 1 SYRINGE (58160-815-43)
| Marketing Start Date | 2007-06-07 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 58160-815-11 [58160081511]
TWINRIX INJECTION, SUSPENSION
| Marketing Category | BLA |
| Application Number | BLA103850 |
| Product Type | VACCINE |
| Billing Unit | ML |
| Marketing Start Date | 2007-06-07 |
| Marketing End Date | 2018-01-09 |
NDC 58160-815-01 [58160081501]
TWINRIX INJECTION, SUSPENSION
| Marketing Category | BLA |
| Application Number | BLA103850 |
| Product Type | VACCINE |
| Billing Unit | ML |
| Marketing Start Date | 2007-06-07 |
| Marketing End Date | 2018-01-09 |
NDC 58160-815-43 [58160081543]
TWINRIX INJECTION, SUSPENSION
| Marketing Category | BLA |
| Application Number | BLA103850 |
| Product Type | VACCINE |
| Billing Unit | ML |
| Marketing Start Date | 2010-09-24 |
NDC 58160-815-34 [58160081534]
TWINRIX INJECTION, SUSPENSION
| Marketing Category | BLA |
| Application Number | BLA103850 |
| Product Type | VACCINE |
| Billing Unit | ML |
| Marketing Start Date | 2011-01-14 |
| Marketing End Date | 2018-01-09 |
NDC 58160-815-41 [58160081541]
TWINRIX INJECTION, SUSPENSION
| Marketing Category | BLA |
| Application Number | BLA103850 |
| Product Type | VACCINE |
| Marketing Start Date | 2007-06-07 |
| Marketing End Date | 2013-12-10 |
NDC 58160-815-05 [58160081505]
TWINRIX INJECTION, SUSPENSION
| Marketing Category | BLA |
| Application Number | BLA103850 |
| Product Type | VACCINE |
| Billing Unit | ML |
| Marketing Start Date | 2012-08-20 |
| Marketing End Date | 2018-01-09 |
NDC 58160-815-48 [58160081548]
TWINRIX INJECTION, SUSPENSION
| Marketing Category | BLA |
| Application Number | BLA103850 |
| Product Type | VACCINE |
| Marketing Start Date | 2010-09-24 |
| Marketing End Date | 2013-12-10 |
NDC 58160-815-52 [58160081552]
TWINRIX INJECTION, SUSPENSION
| Marketing Category | BLA |
| Application Number | BLA103850 |
| Product Type | VACCINE |
| Billing Unit | ML |
| Marketing Start Date | 2011-01-07 |
NDC 58160-815-32 [58160081532]
TWINRIX INJECTION, SUSPENSION
| Marketing Category | BLA |
| Application Number | BLA103850 |
| Product Type | VACCINE |
| Billing Unit | ML |
| Marketing Start Date | 2009-11-21 |
| Marketing End Date | 2011-10-09 |
NDC 58160-815-46 [58160081546]
TWINRIX INJECTION, SUSPENSION
| Marketing Category | BLA |
| Application Number | BLA103850 |
| Product Type | VACCINE |
| Marketing Start Date | 2007-06-07 |
| Marketing End Date | 2013-12-10 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| HEPATITIS A VIRUS STRAIN HM175 ANTIGEN (FORMALDEHYDE INACTIVATED) | 720 [iU]/mL |
Pharmacological Class
- Inactivated Hepatitis A Virus Vaccine [EPC]
- Actively Acquired Immunity [PE]
- Hepatitis A Vaccines [CS]
- Vaccines
- Inactivated [CS]
NDC Crossover Matching brand name "TWINRIX" or generic name "Hepatitis A And Hepatitis B (recombinant) Vaccine"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 50090-1645 | TWINRIX | Hepatitis A and Hepatitis B (Recombinant) Vaccine |
| 58160-815 | TWINRIX | Hepatitis A and Hepatitis B (Recombinant) Vaccine |
Trademark Results [TWINRIX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TWINRIX 76534857 not registered Dead/Abandoned |
GlaxoSmithKline Biologicals S.A. 2003-08-04 |
 TWINRIX 76534054 not registered Dead/Abandoned |
GlaxoSmithKline Biologicals S.A. 2003-08-01 |
 TWINRIX 75819718 not registered Dead/Abandoned |
SmithKline Beecham Biologicals S.A. 1999-10-06 |
 TWINRIX 75819717 not registered Dead/Abandoned |
SmithKline Beecham Biologicals S.A. 1999-10-06 |
 TWINRIX 75819716 not registered Dead/Abandoned |
SmithKline Beecham Biologicals S.A. 1999-10-06 |
 TWINRIX 75819715 not registered Dead/Abandoned |
SmithKline Beecham Biologicals S.A. 1999-10-06 |
 TWINRIX 75819714 not registered Dead/Abandoned |
SmithKline Beecham Biologicals S.A. 1999-10-06 |
 TWINRIX 75819713 2585955 Dead/Cancelled |
SmithKline Beecham Biologicals S.A. 1999-10-06 |
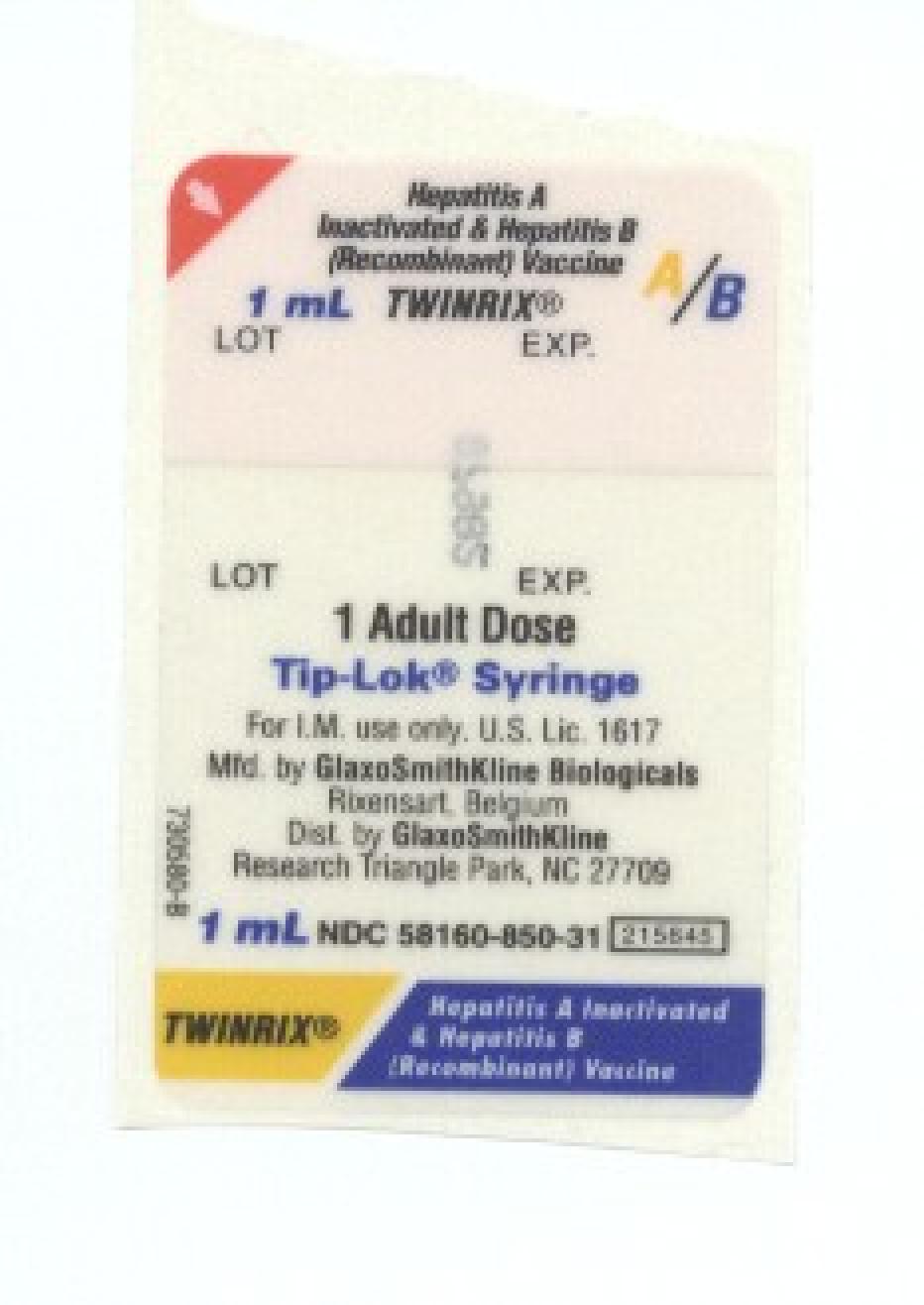 TWINRIX 75456748 2240725 Live/Registered |
GlaxoSmithKline Biologicals S.A 1998-03-26 |
 TWINRIX 74582417 not registered Dead/Abandoned |
SmithKline Beecham Biologicals S.A. 1994-10-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.