NDC 58264-0106
D-100
Fucus Vesiculosus
D-100 is a Sublingual Solution in the Human Otc Drug category. It is labeled and distributed by Dna Labs, Inc.. The primary component is Fucus Vesiculosus.
| Product ID | 58264-0106_dc71203a-e92b-480c-8fdb-69b55cdc69ee |
| NDC | 58264-0106 |
| Product Type | Human Otc Drug |
| Proprietary Name | D-100 |
| Generic Name | Fucus Vesiculosus |
| Dosage Form | Solution |
| Route of Administration | SUBLINGUAL |
| Marketing Start Date | 1990-01-01 |
| Marketing Category | UNAPPROVED HOMEOPATHIC / UNAPPROVED HOMEOPATHIC |
| Labeler Name | DNA Labs, Inc. |
| Substance Name | FUCUS VESICULOSUS |
| Active Ingredient Strength | 3 [hp_X]/mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 58264-0106-1
29.57 mL in 1 BOTTLE, GLASS (58264-0106-1)
| Marketing Start Date | 1990-01-01 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 58264-0106-1 [58264010601]
D-100 SOLUTION
| Marketing Category | UNAPPROVED HOMEOPATHIC |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 1990-01-01 |
| Inactivation Date | 2020-01-31 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| FUCUS VESICULOSUS | 3 [hp_X]/mL |
OpenFDA Data
| SPL SET ID: | 0a2aed6f-6e1c-43e4-b5df-1dc73d7e6f2b |
| Manufacturer | |
| UNII |
NDC Crossover Matching brand name "D-100" or generic name "Fucus Vesiculosus"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 58264-0106 | D-100 | Fucus vesiculosus |
| 0220-2165 | Fucus vesiculosus | FUCUS VESICULOSUS |
| 0220-2167 | Fucus vesiculosus | FUCUS VESICULOSUS |
| 15631-0199 | FUCUS VESICULOSUS | FUCUS VESICULOSUS |
| 15631-0587 | FUCUS VESICULOSUS | FUCUS VESICULOSUS |
| 60512-6695 | FUCUS VESICULOSUS | FUCUS VESICULOSUS |
| 68428-944 | Fucus vesiculosus | FUCUS VESICULOSUS |
| 71919-304 | Fucus vesiculosus | FUCUS VESICULOSUS |
Trademark Results [D-100]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
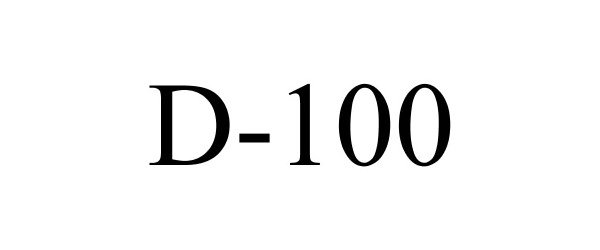 D-100 85850614 4993699 Live/Registered |
Bio-Rad Laboratories, Inc. 2013-02-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.