NDC 58443-0370
Sky Wellness Max Pain Relief
Lidocaine Hydrochloride, Menthol
Sky Wellness Max Pain Relief is a Topical Stick in the Human Otc Drug category. It is labeled and distributed by Prime Enterprises. The primary component is Lidocaine Hydrochloride; Menthol.
| Product ID | 58443-0370_b1a64641-842c-17a8-e053-2a95a90a2007 |
| NDC | 58443-0370 |
| Product Type | Human Otc Drug |
| Proprietary Name | Sky Wellness Max Pain Relief |
| Generic Name | Lidocaine Hydrochloride, Menthol |
| Dosage Form | Stick |
| Route of Administration | TOPICAL |
| Marketing Start Date | 2019-05-01 |
| Marketing Category | OTC MONOGRAPH NOT FINAL / OTC MONOGRAPH NOT FINAL |
| Application Number | part348 |
| Labeler Name | Prime Enterprises |
| Substance Name | LIDOCAINE HYDROCHLORIDE; MENTHOL |
| Active Ingredient Strength | 38 mg/mL; mg/mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2021-12-31 |
Packaging
NDC 58443-0370-3
89 mL in 1 BOTTLE (58443-0370-3)
| Marketing Start Date | 2019-05-01 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "Sky Wellness Max Pain Relief" or generic name "Lidocaine Hydrochloride, Menthol"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 71868-080 | Activate Joint and Muscle Relief | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 69418-002 | BenePatch | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 58443-0403 | Botanika Life | Lidocaine Hydrochloride, Menthol |
| 72614-266 | CANNA NUMB Pain Relief | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 72614-265 | CANNA NUMB Roll On | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 10738-207 | CBD PAIN RELIEF Cream | Lidocaine Hydrochloride, Menthol |
| 58443-0366 | CBDaF | Lidocaine Hydrochloride, Menthol |
| 72465-600 | INSTA RELIEF DM | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 58443-0336 | JOIYA Ease | lidocaine Hydrochloride, Menthol |
| 53217-026 | LenzaGel | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 63187-161 | LenzaGel | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 68788-9672 | LenzaGel | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 63187-162 | LenzaPatch | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 63187-892 | Lidozen | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 63187-917 | Lidozen | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 71205-302 | Lidozen | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 71574-300 | Lidozen | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 71574-400 | Lidozen | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 71574-800 | Lidozen | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 71205-715 | Lidozen Gel | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 71574-305 | Lidozen Gel | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 72816-000 | Mamisan Pain Relieving | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 76102-031 | Maximum Strength Pain Relief | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 72188-170 | MAXOCAINE PAIN RELIEVING ROLL-ON | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 69889-026 | MTX Topical Pain | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 58443-0268 | Performance Brands Steel Releaf | Lidocaine Hydrochloride, Menthol |
| 82798-295 | RAPID PAIN-RELIEF TOPICAL | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 69418-006 | Ricora | LIDOCAINE HYDROCHLORIDE, MENTHOL |
| 58443-0370 | Sky Wellness | Lidocaine Hydrochloride, Menthol |
| 76420-206 | Zylotrol Pain Relieving | LIDOCAINE HYDROCHLORIDE, MENTHOL |
Trademark Results [Sky Wellness]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
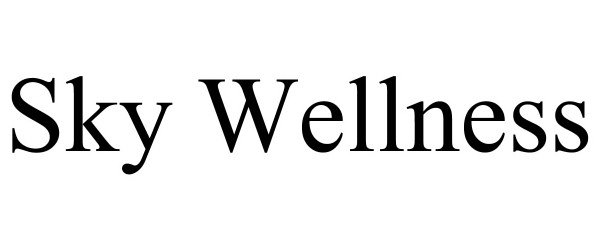 SKY WELLNESS 90828919 not registered Live/Pending |
Sky Wellness, LLC 2021-07-14 |
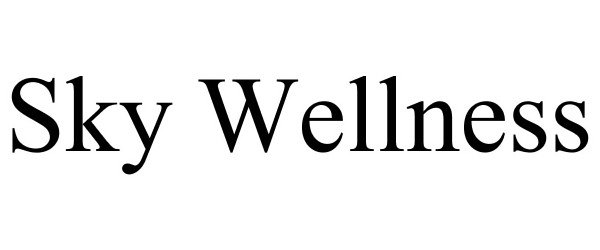 SKY WELLNESS 87914715 not registered Live/Pending |
Beacon Management, LLC. 2018-05-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.