NDC 59011-271
Hysingla ER
Hydrocodone Bitartrate
Hysingla ER is a Oral Tablet, Extended Release in the Human Prescription Drug category. It is labeled and distributed by Purdue Pharma Lp. The primary component is Hydrocodone Bitartrate.
| Product ID | 59011-271_1f1ceab3-0084-d944-a84f-1344873c39fd |
| NDC | 59011-271 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Hysingla ER |
| Generic Name | Hydrocodone Bitartrate |
| Dosage Form | Tablet, Extended Release |
| Route of Administration | ORAL |
| Marketing Start Date | 2015-01-15 |
| Marketing Category | NDA / NDA |
| Application Number | NDA206627 |
| Labeler Name | Purdue Pharma LP |
| Substance Name | HYDROCODONE BITARTRATE |
| Active Ingredient Strength | 20 mg/1 |
| Pharm Classes | Opioid Agonist [EPC],Opioid Agonists [MoA] |
| DEA Schedule | CII |
| NDC Exclude Flag | N |
| Listing Certified Through | 2021-12-31 |
Packaging
NDC 59011-271-60
60 TABLET, EXTENDED RELEASE in 1 BOTTLE, PLASTIC (59011-271-60)
| Marketing Start Date | 2015-01-15 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 59011-271-60 [59011027160]
Hysingla ER TABLET, EXTENDED RELEASE
| Marketing Category | NDA |
| Application Number | NDA206627 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2015-01-15 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| HYDROCODONE BITARTRATE | 20 mg/1 |
OpenFDA Data
| SPL SET ID: | b7d23ac2-e776-9f62-3290-c64c2d6eb353 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI | |
| UPC Code |
Pharmacological Class
- Opioid Agonist [EPC]
- Opioid Agonists [MoA]
Medicade Reported Pricing
59011027160 HYSINGLA ER 20 MG TABLET
Pricing Unit: EA | Drug Type:
NDC Crossover Matching brand name "Hysingla ER" or generic name "Hydrocodone Bitartrate"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 59011-276 | Hysingla ER | Hysingla ER |
| 59011-272 | Hysingla ER | Hysingla ER |
| 59011-273 | Hysingla ER | Hysingla ER |
| 59011-275 | Hysingla ER | Hysingla ER |
| 59011-274 | Hysingla ER | Hysingla ER |
| 59011-277 | Hysingla ER | Hysingla ER |
| 59011-271 | Hysingla ER | Hysingla ER |
| 47781-392 | Hydrocodone Bitartrate | Hydrocodone Bitartrate |
| 47781-393 | Hydrocodone Bitartrate | Hydrocodone Bitartrate |
| 47781-394 | Hydrocodone Bitartrate | Hydrocodone Bitartrate |
| 47781-395 | Hydrocodone Bitartrate | Hydrocodone Bitartrate |
| 47781-396 | Hydrocodone Bitartrate | Hydrocodone Bitartrate |
| 47781-397 | Hydrocodone Bitartrate | Hydrocodone Bitartrate |
| 47781-398 | Hydrocodone Bitartrate | Hydrocodone Bitartrate |
| 47781-409 | Hydrocodone Bitartrate | Hydrocodone Bitartrate |
| 47781-410 | Hydrocodone Bitartrate | Hydrocodone Bitartrate |
| 47781-411 | Hydrocodone Bitartrate | Hydrocodone Bitartrate |
| 47781-412 | Hydrocodone Bitartrate | Hydrocodone Bitartrate |
| 47781-413 | Hydrocodone Bitartrate | Hydrocodone Bitartrate |
| 47781-414 | Hydrocodone Bitartrate | Hydrocodone Bitartrate |
| 43376-210 | Zohydro | hydrocodone bitartrate |
| 43376-215 | Zohydro | hydrocodone bitartrate |
| 43376-220 | Zohydro | hydrocodone bitartrate |
| 43376-230 | Zohydro | hydrocodone bitartrate |
| 43376-240 | Zohydro | hydrocodone bitartrate |
| 43376-250 | Zohydro | hydrocodone bitartrate |
| 43376-310 | Zohydro | hydrocodone bitartrate |
| 43376-315 | Zohydro | hydrocodone bitartrate |
| 43376-320 | Zohydro | hydrocodone bitartrate |
| 43376-330 | Zohydro | hydrocodone bitartrate |
| 43376-340 | Zohydro | hydrocodone bitartrate |
| 43376-350 | Zohydro | hydrocodone bitartrate |
Trademark Results [Hysingla ER]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HYSINGLA ER 86446650 not registered Dead/Abandoned |
Purdue Pharma L.P. 2014-11-06 |
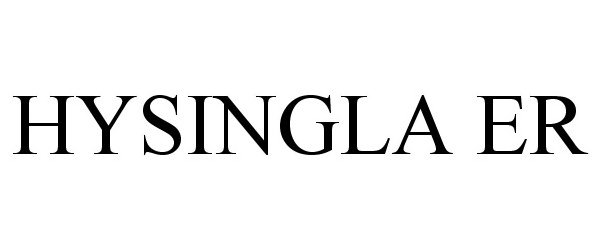 HYSINGLA ER 86242700 4713945 Live/Registered |
Purdue Pharma L.P. 2014-04-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.