NDC 59088-452
Lidogel
Lidocaine Hci
Lidogel is a Topical Gel in the Human Otc Drug category. It is labeled and distributed by Puretek Corporation. The primary component is Lidocaine Hydrochloride.
| Product ID | 59088-452_c2e13ea2-7d2b-ebf8-e053-2995a90a474a |
| NDC | 59088-452 |
| Product Type | Human Otc Drug |
| Proprietary Name | Lidogel |
| Generic Name | Lidocaine Hci |
| Dosage Form | Gel |
| Route of Administration | TOPICAL |
| Marketing Start Date | 2021-07-08 |
| Marketing Category | OTC MONOGRAPH NOT FINAL / |
| Application Number | part348 |
| Labeler Name | PureTek Corporation |
| Substance Name | LIDOCAINE HYDROCHLORIDE |
| Active Ingredient Strength | 28 mg/g |
| NDC Exclude Flag | N |
| Listing Certified Through | 2022-12-31 |
Packaging
NDC 59088-452-07
100 g in 1 TUBE (59088-452-07)
| Marketing Start Date | 2021-07-08 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "Lidogel" or generic name "Lidocaine Hci"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 59088-452 | Lidogel | Lidocaine HCI |
| 59088-466 | Lidogel | Lidocaine HCl |
| 69396-106 | Burn Gel | Lidocaine HCI |
| 53200-001 | Derma Numb | Lidocaine HCI |
| 51662-1230 | LIDOCAINE HCI | LIDOCAINE HCI |
| 51662-1232 | LIDOCAINE HCI | LIDOCAINE HCI |
| 51662-1233 | LIDOCAINE HCI | LIDOCAINE HCI |
| 51662-1364 | LIDOCAINE HCI | LIDOCAINE HCI |
| 51662-1382 | LIDOCAINE HCI | LIDOCAINE HCI |
| 51662-1384 | LIDOCAINE HCI | LIDOCAINE HCI |
| 51662-1389 | LIDOCAINE HCI | LIDOCAINE HCI |
| 51662-1416 | LIDOCAINE HCI | LIDOCAINE HCI |
| 51662-1419 | LIDOCAINE HCI | LIDOCAINE HCI |
| 51662-1422 | LIDOCAINE HCI | LIDOCAINE HCI |
| 51662-1461 | LIDOCAINE HCI | LIDOCAINE HCI |
| 51662-1543 | LIDOCAINE HCI | LIDOCAINE HCI |
| 11822-1115 | Pain Relief | Lidocaine HCI |
| 41250-779 | Pain Relief | Lidocaine HCI |
| 49035-149 | Pain Relief | Lidocaine HCI |
| 69842-222 | Pain Relief | Lidocaine HCI |
| 0363-7780 | Pain Relief Roll-on | Lidocaine HCI |
Trademark Results [Lidogel]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
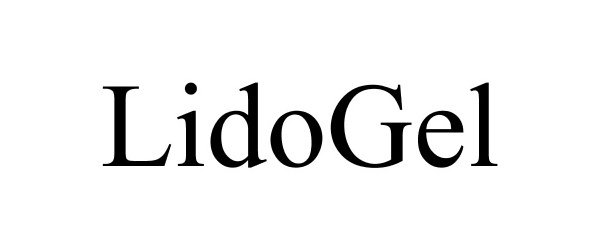 LIDOGEL 85455516 not registered Dead/Abandoned |
Gensco Laboratories, LLC 2011-10-25 |
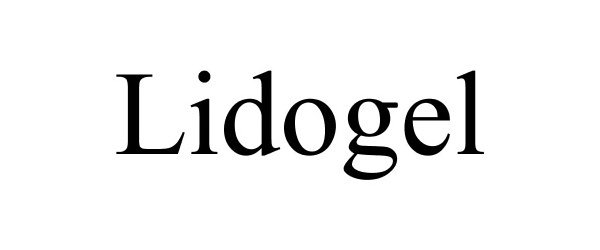 LIDOGEL 85455493 not registered Dead/Abandoned |
Gensco Laboratories, LLC 2011-10-25 |
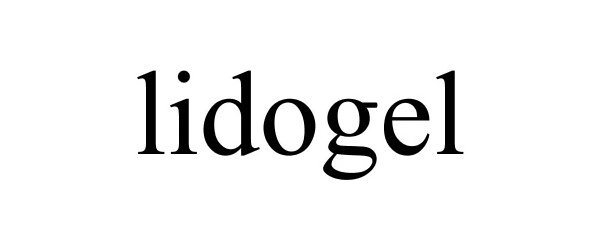 LIDOGEL 77071025 not registered Dead/Abandoned |
Nickell, Robert P 2006-12-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.