NDC 59779-874
allergy relief childrens
Diphenhydramine Hcl
allergy relief childrens is a Oral Solution in the Human Otc Drug category. It is labeled and distributed by Cvs Pharmacy. The primary component is Diphenhydramine Hydrochloride.
| Product ID | 59779-874_748aacd4-c3c2-43b1-a417-1b33eec41168 |
| NDC | 59779-874 |
| Product Type | Human Otc Drug |
| Proprietary Name | allergy relief childrens |
| Generic Name | Diphenhydramine Hcl |
| Dosage Form | Solution |
| Route of Administration | ORAL |
| Marketing Start Date | 2015-09-03 |
| Marketing Category | OTC MONOGRAPH FINAL / OTC MONOGRAPH FINAL |
| Application Number | part341 |
| Labeler Name | CVS Pharmacy |
| Substance Name | DIPHENHYDRAMINE HYDROCHLORIDE |
| Active Ingredient Strength | 13 mg/5mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2020-12-31 |
Packaging
NDC 59779-874-26
1 BOTTLE in 1 CARTON (59779-874-26) > 118 mL in 1 BOTTLE
| Marketing Start Date | 2015-09-03 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 59779-874-26 [59779087426]
allergy relief childrens SOLUTION
| Marketing Category | OTC monograph final |
| Application Number | part341 |
| Product Type | HUMAN OTC DRUG |
| Billing Unit | ML |
| Marketing Start Date | 2015-09-03 |
NDC 59779-874-34 [59779087434]
allergy relief childrens SOLUTION
| Marketing Category | OTC monograph final |
| Application Number | part341 |
| Product Type | HUMAN OTC DRUG |
| Billing Unit | ML |
| Marketing Start Date | 2015-09-03 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| DIPHENHYDRAMINE HYDROCHLORIDE | 12.5 mg/5mL |
OpenFDA Data
| SPL SET ID: | 8b539dc0-5487-4b11-babd-52bf341a8947 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
NDC Crossover Matching brand name "allergy relief childrens" or generic name "Diphenhydramine Hcl"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 68016-051 | Allergy Relief Childrens | Allergy Relief Childrens |
| 33992-0292 | Allergy Relief Childrens | Allergy Relief Childrens |
| 37808-092 | Allergy Relief Childrens | Allergy Relief Childrens |
| 37808-085 | Allergy Relief Childrens | Allergy Relief Childrens |
| 41163-585 | allergy relief Childrens | allergy relief Childrens |
| 41250-599 | Allergy Relief Childrens | Allergy Relief Childrens |
| 59779-837 | allergy relief childrens | allergy relief childrens |
| 59779-874 | allergy relief childrens | allergy relief childrens |
| 59779-884 | Allergy Relief Childrens | Allergy Relief Childrens |
| 46122-425 | Allergy Relief Childrens | Allergy Relief Childrens |
| 0113-7379 | basic care childrens allergy relief | Diphenhydramine HCl |
| 0113-7186 | basic care nighttime sleep aid | Diphenhydramine HCl |
| 0113-7506 | basic care nighttime sleep aid | diphenhydramine hcl |
| 0363-0481 | Childrens Allergy Dye-Free Wal Dryl | Diphenhydramine HCl |
| 0113-0379 | good sense allergy | Diphenhydramine HCl |
| 0113-0052 | good sense sleep time | Diphenhydramine HCl |
| 0113-0186 | Good Sense Sleep Time | Diphenhydramine HCl |
| 0363-0353 | Itch Relief Gel | Diphenhydramine HCl |
| 0363-0236 | Nighttime Sleep Aid | Diphenhydramine HCl |
| 0363-0367 | Sleep Aid | Diphenhydramine HCl |
| 0363-0189 | Sleep II | Diphenhydramine HCl |
| 0363-0190 | Wal-Dryl | Diphenhydramine HCl |
| 0363-0418 | Wal-Dryl | Diphenhydramine HCl |
| 0363-0329 | WAL-DRYL ALLERGY | Diphenhydramine HCl |
| 0363-0020 | Wal-Sleep Z | Diphenhydramine HCl |
Trademark Results [allergy relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
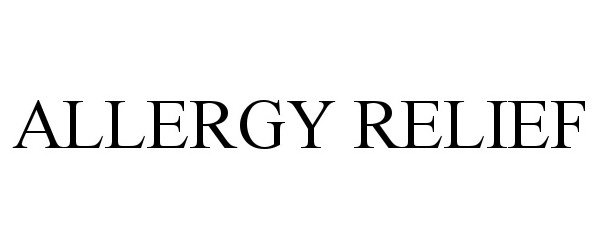 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
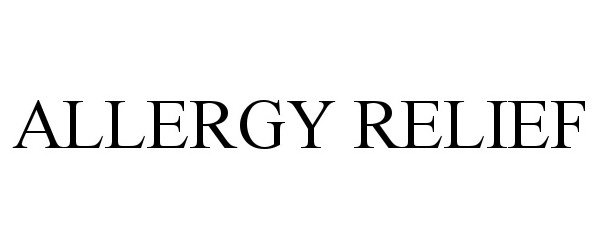 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
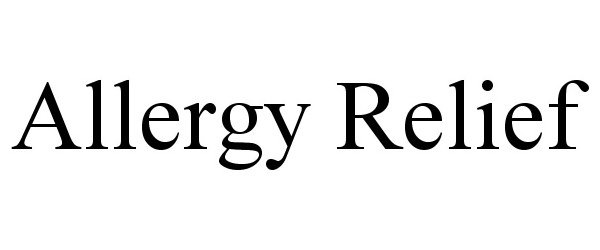 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
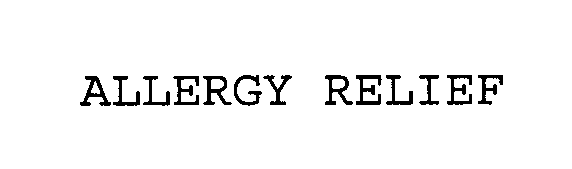 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.