NDC 62856-797
Aloxi
Palonosetron Hydrochloride
Aloxi is a Intravenous Injection in the Human Prescription Drug category. It is labeled and distributed by Eisai Inc.. The primary component is Palonosetron Hydrochloride.
| Product ID | 62856-797_afcfa291-df05-4e5a-9976-87b0cc8bd378 |
| NDC | 62856-797 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Aloxi |
| Generic Name | Palonosetron Hydrochloride |
| Dosage Form | Injection |
| Route of Administration | INTRAVENOUS |
| Marketing Start Date | 2014-05-28 |
| Marketing End Date | 2022-10-31 |
| Marketing Category | NDA / NDA |
| Application Number | NDA021372 |
| Labeler Name | Eisai Inc. |
| Substance Name | PALONOSETRON HYDROCHLORIDE |
| Active Ingredient Strength | 0 mg/5mL |
| Pharm Classes | Serotonin 3 Receptor Antagonists [MoA],Serotonin-3 Receptor Antagonist [EPC] |
| NDC Exclude Flag | N |
Packaging
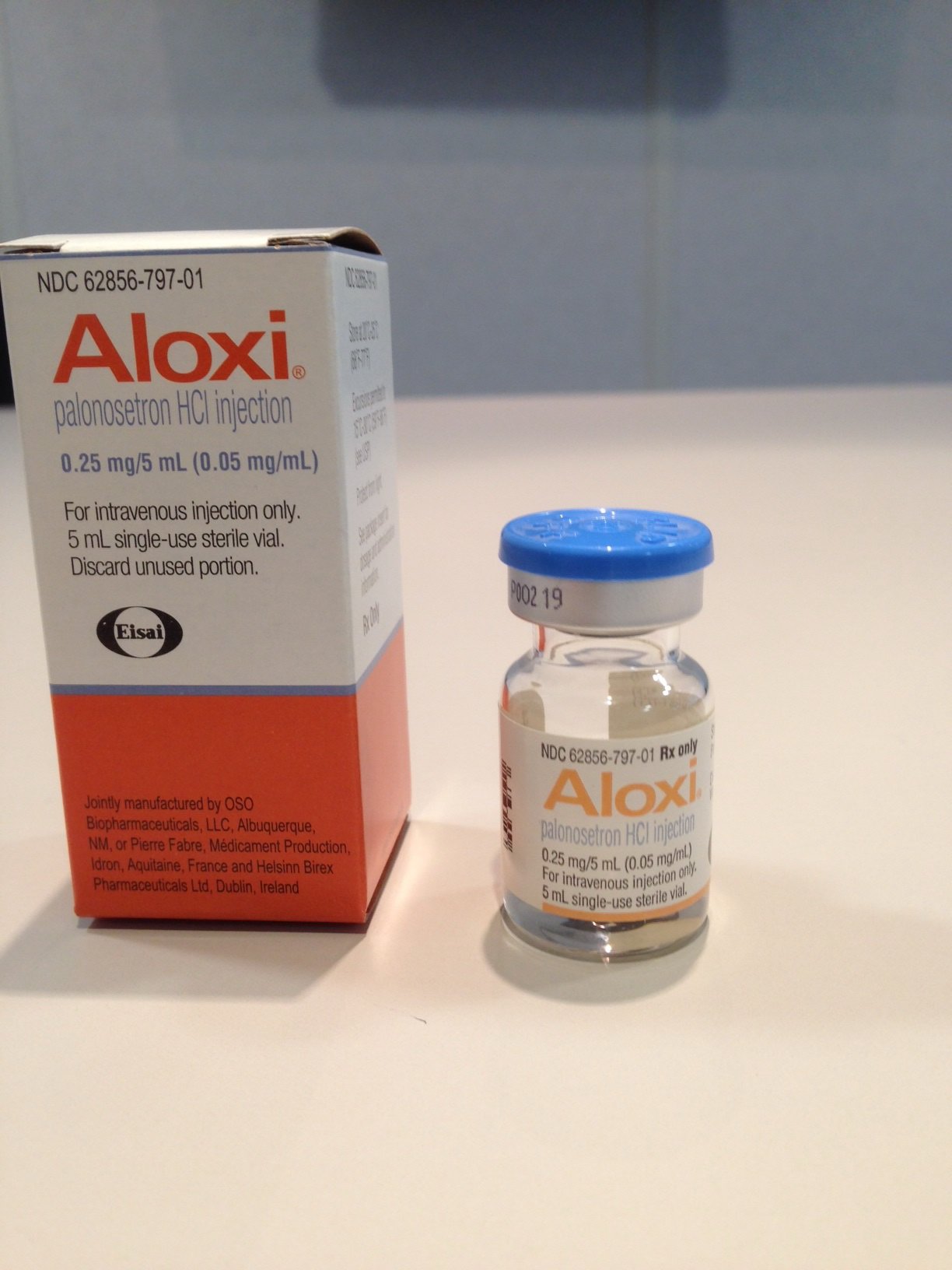
NDC 62856-797-01
1 VIAL in 1 CARTON (62856-797-01) > 5 mL in 1 VIAL
| Marketing Start Date | 2014-05-28 |
| Marketing End Date | 2022-10-31 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 62856-797-01 [62856079701]
Aloxi INJECTION
| Marketing Category | NDA |
| Application Number | NDA021372 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | ML |
| Marketing Start Date | 2014-05-28 |
| Inactivation Date | 2020-01-31 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| PALONOSETRON HYDROCHLORIDE | .25 mg/5mL |
OpenFDA Data
| SPL SET ID: | 1b51c34d-9631-4520-83af-6f6fb21a58a4 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI | |
| UPC Code |
Pharmacological Class
- Serotonin 3 Receptor Antagonists [MoA]
- Serotonin-3 Receptor Antagonist [EPC]
NDC Crossover Matching brand name "Aloxi" or generic name "Palonosetron Hydrochloride"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 62856-797 | Aloxi | palonosetron hydrochloride |
| 62856-798 | Aloxi | palonosetron hydrochloride |
| 69639-103 | Aloxi | Aloxi |
| 69639-104 | ALOXI | ALOXI |
| 60505-6193 | Palonosetron | Palonosetron Hydrochloride |
| 67184-0514 | Palonosetron | Palonosetron Hydrochloride |
| 67184-0515 | Palonosetron | Palonosetron Hydrochloride |
| 0143-9513 | Palonosetron Hydrochloride | Palonosetron Hydrochloride |
| 0409-2504 | Palonosetron Hydrochloride | PALONOSETRON HYDROCHLORIDE |
| 0703-4094 | Palonosetron Hydrochloride | Palonosetron Hydrochloride |
| 0781-3312 | Palonosetron Hydrochloride | Palonosetron Hydrochloride |
| 0781-3415 | Palonosetron Hydrochloride | Palonosetron Hydrochloride |
| 16729-365 | palonosetron hydrochloride | palonosetron hydrochloride |
| 23155-356 | Palonosetron Hydrochloride | Palonosetron Hydrochloride |
| 25021-783 | palonosetron hydrochloride | palonosetron hydrochloride |
| 25021-788 | palonosetron hydrochloride | palonosetron hydrochloride |
| 36000-326 | Palonosetron Hydrochloride | palonosetron hydrochloride |
| 50742-485 | Palonosetron Hydrochloride | Palonosetron Hydrochloride |
| 55150-186 | Palonosetron Hydrochloride | Palonosetron Hydrochloride |
| 67457-317 | Palonosetron Hydrochloride | Palonosetron Hydrochloride |
Trademark Results [Aloxi]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALOXI 98017461 not registered Live/Pending |
Ingenus Pharmaceuticals, LLC 2023-05-29 |
 ALOXI 86911254 5144689 Live/Registered |
Helsinn Healthcare SA 2016-02-17 |
 ALOXI 86488666 not registered Indifferent |
Helsinn Healthcare SA 0000-00-00 |
 ALOXI 86488652 4897369 Live/Registered |
Helsinn Healthcare SA 2014-12-22 |
 ALOXI 76377426 2863255 Live/Registered |
Helsinn Healthcare SA 2002-03-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.